Creating A New Standard For Treating CNS Disease
Inhibikase Therapeutics (NASDAQ:IKT) is a biotechnology company focused on creating new therapies for Central Nervous System (CNS) based diseases. The company has concentrated its efforts on developing small-molecule kinase inhibitor therapeutics for the safe and effective treatment of neurological diseases.
The company has focused its research and development efforts on various neurological disorders and believes that it can change how the current therapies are being carried out while simultaneously improving the quality of life.
Company’s Product Pipeline
Inhibikase has a multi-Indication pipeline encompassing Neurodegeneration, Oncology, and Infectious disease. The company has developed product candidates using its RAMP technology that uses pre-existing chemical entities as templates to develop novel small molecule kinase Inhibitors, to make sure that the safety profile of the novel drug matches the template.
kT-148009 for Parkinson’s Disease
The company’s lead small molecule drug candidate, IkT-148009, is an Abelson tyrosine kinase inhibitor (c-Abl inhibitor) that stops the progression of Parkinson’s disease and reverses the functional loss due to disease in the brain, as well as its manifestation in the Gastrointestinal (GI) tract.
The company started with the Phase 1 trial in February 2021. Early results from the trial indicate optimistic results. Early results from the trial indicate optimistic results as a 25 mg once daily oral dose in older and elderly healthy subjects in the Phase 1 study reached exposure which were consistent with the results seen in the preclinical stage. The Phase 1/Phase 2 development program, subject to FDA approval, would be followed by the Phase 3 clinical trial that is expected to conclude the clinical development program in either 2023 or 2024.
IkT-001 Pro for Chronic Myeloid Leukemia (CML)
The Company’s second small molecule drug candidate, IkT-001 Pro, is a prodrug of the anti-cancer agent Imatinib (an orphan drug), an FDA-approved treatment for certain blood and stomach cancers. A prodrug is a medication that the body metabolizes into an active drug after it is administered. Prodrugs can be used to improve drug delivery, decrease toxicity, or target the drug to specific cells.
The pre-clinical development results indicate that evaluation of the prodrug absorption and distribution in rats demonstrated that the exposure to Imatinib is significantly high overall. The study also suggested that the prodrug suppressed specific side effects that generally arise from Imatinib. The company expects to file an IND to initiate a clinical trial for Ikt-001Pro by the third Quarter of 2021 and expects the clinical development to be complete by the end of 2022.
Other Drug Candidates
The Company’s RAMP has identified additional opportunities for other neurological diseases, which include Dementia with Lewy Body (DLB), Multiple System Atrophy (MSA), and Progressive Multifocal Leukoencephalopathy (PML). These are complex neurological disorders with limited research towards potential treatments.
How does IkT-148009 work?
IkT-148009 is a protein kinase inhibitor to treat Parkinson’s disease and the complications arising from it. Parkinson’s disease is initiated by a dysfunctional protein known as alpha-synuclein.
Abelson tyrosine kinase plays an essential role in the disease process of Parkinson’s. c-Abl chemically modifies one of the proteins in PD, known as alpha-synuclein. c-Abl activation also leads to modification of a second protein, parkin. Parkin usually tags toxic proteins like dysfunctional alpha-synuclein to remove them through an enzyme process known as the proteasome, which is the survival pathway that usually protects neurons from toxic proteins.
But when c-Abl acts on parkin, it inactivates the protein leading to the shutting down of the survival pathway. IkT-148009 acts on c-Abl to inhibit the activation of the protein, which leads to the removal of dysfunctional alpha-synuclein, leading to the reversal of the impact of Parkinson’s disease.
Current Treatment Versus IkT-148009
There is no cure for Parkinson’s disease currently, but PD patients can use a few approved medications. Levodopa is the most commonly prescribed medicine for Parkinson’s disease. It helps control the symptoms like slow movements and stiff and rigid body parts by increasing dopamine levels in the brain. There are a few side effects of the drug which include vomiting, nausea, and confusion. People who take levodopa for 3-5 years might develop symptoms like restlessness, unusual movement, or dyskinesia within a few hours of taking medication.
There are many other medications like Amantadine, COMT inhibitors, MAO-B inhibitors, but these all have side effects which adversely affect the quality of life. On the other hand, IkT-148009 is expected to modify the disease, slowing or stopping progression and restoring some or much of the functional loss.
Parkinson’s Disease Is A Huge Market
Parkinson’s disease is one of the world’s fastest-growing neurological disorders. It is a slowly progressive neurodegenerative disorder that causes debilitating symptoms like tremors, bradykinesia, anxiety, depression, and cognitive impairments. People with PD often experience significant comorbidities, including increased infections, cardiac and gastrointestinal disorders, and fall-related injuries.
Parkinson’s disease is the second most common age-related neurodegenerative disorder after Alzheimer’s disease. An estimated ten million people globally suffer from Parkinson’s disease. PD’s prevalence ranges from 41 people per 100,000 who are 40 and older to more than 1,900 people per 100,000 among those who are 80 and older.
According to Fortune Business Insights, the Global Parkinson’s disease treatment market was valued at $4.50 billion in 2018 and is expected to reach $8.38 billion by the end of 2026, exhibiting a CAGR of 8.1%.
Decarboxylase inhibitors, which consist of carbidopa and levodopa, dominate the PD treatment market with a share of 37.8%; however, it has been observed that this medication is not as effective as originally intended. Therefore, drugs that use novel methods for effective treatment are actively being researched by pharmaceutical companies across the world.
Economic Impact Of Parkinson Disease
According to the National Center for Biotechnology Information (NCBI), PD incurred a total economic burden of $51.9 billion in 2017. The total burden of PD includes direct medical costs of $25.4 billion and $26.5 billion in indirect and non-medical costs.
It is estimated that the projected total economic burden will surpass $79 billion by the year 2037, out of which $40.4 billion will be directly attributed to “Direct Medical” costs. Inhibikase believes that its lead small molecule drug candidate, IkT-148009, can be more effective than the current treatments available, thus making Inhibikase a direct beneficiary of the rapidly expanding PD treatment market.
Competitive Landscape
Generally, there are two approaches of medication to a particular disease; one is to relieve the symptoms of the disease, and the second is to modify the disease itself. Inhibikase Therapeutics takes the latter approach, developing drugs that are intended to modify the disease.
All the current treatments available in the market only help to relieve the symptoms, and none of the treatments affect the progression of the disease. If Inhibikase’s novel treatment approach is approved, it can be a game-changer for the PD treatment market and could easily capture a large segment of the market share.
However, there are several more companies that are working to develop a novel drug for the treatment of PD, which could heavily compete with Inhibikase.
There are three main strategies that are being researched to treat PD. They are:
1. Alpha-synuclein strategies
Preventing α-synuclein aggregation and dissolving pre-formed aggregates may be an effective strategy for treating PD. The following drugs are currently being investigated to treat PD from this angle.
|
Drug Name |
Company Name |
Phase |
|
Prasinezumab |
Hoffman-La Roche |
Phase 2 |
|
MEDI1341 |
AstraZeneca (NASDAQ:AZN) |
Phase 1 |
|
LU AF82422 |
H. Lundbeck |
Phase 1 |
|
ABBV-0805 |
AbbVie (NYSE:ABBV) |
Phase 1 |
2. LRRK2 strategies
LRRK2 is a gene that causes autosomal dominant PD. The gene encodes the LRRK2 enzyme, which adds phosphate groups onto other proteins. Mutations in LRRK2 cause PD to increase the activity of LRRK2. There is evidence that LRRK2 acts to regulate alpha-synuclein as well as neuroinflammation. The following drugs are currently being investigated to treat PD from this angle.
|
Drug Name |
Company Name |
Phase |
|
DNL151 |
Denali Therapeutics (NASDAQ:DNLI) |
Phase 1 |
|
DNL201 |
Denali Therapeutics |
Phase 1 |
|
BIIB094 |
Biogen (NASDAQ:BIIB) |
Phase 1 |
3. c-Abl kinase inhibitor strategies
Research suggests that overactivation of c-Abl is a downstream effect of oxidative stress and may accelerate neurodegeneration in PD. There is also research to suggest that c-Abl activation correlates with alpha-synuclein aggregation. These findings and others led to the possibility that inhibiting c-Abl may be a helpful strategy in PD therapy. Inhibikase Therapeutics aims to use this strategy to treat PD. There are few other companies that are using the same strategy to develop a drug for PD, posing direct competition to Inhibikase.
|
Drug Name |
Company Name |
Phase |
|
K0706 |
Sun Pharma Limited |
Phase 2 |
|
FB-101 |
1ST Biotherapeutics, Inc |
Phase 1 |
We can see that almost all the competitors that are developing a novel treatment for treating PD are currently in Phase 1 trials. Only two drugs, Prasinezumab by Roche and K0706 by Sun Pharma limited, are in Phase 2 trials. Prasinezumab is believed to be a good potential treatment for PD, but it is not a direct competitor to IkT-148009 as it works on a different strategy.
K0706 by Sun Pharma is a direct competitor to IkT as it works on the same strategy and is in an advanced stage of clinical trials. It would be especially noteworthy to see how K0706 performs in future trials.
IP Profile
As of December 2020, the company's patent portfolio includes six issued patents and two pending patent applications in the United States, and three issued patents and ten pending patent applications in foreign markets. The company has received patents that pertain to the composition of matter for IkT-148009, IkT-001 Pro, and IkT-01427.
The IkT-001 Pro patent will expire between 2033 and 2034, whereas for the other two drugs, the patent will expire in 2036.
Company’s Financial Position
The company’s annual cash burn rate had been $1.50 million through 2020. However, with progress in the clinical development of IkT-148009 and other drugs getting approved for the clinical phase, it is now expected to increase to $8-$10 million starting in 2021.
The company’s cash balance is $9.60 million, which would only be sufficient to fund the company's operation for about another year.
Given the company’s arrangement with the U.S. federal government, supporting its research through grants, the risk of extensive share dilution is low for current shareholders, in our opinion.
Valuation Outlook
Of all the drugs expected to treat Parkinson’s disease, IkT-148009 has a market potential of more than $4.50 billion, and IkT-001 Pro, expected to treat CML, has a market potential of $550 million. The likelihood of approval from Phase 1 for all development candidates during 2010-2020 was 7.9%.
On the other hand, drugs taking the 505(b)(2) route are comparatively less costly and have a higher success rate. The company is also focused on developing an effective treatment for PD, which is likely to be significantly better than the current treatment options.
Moreover, the IkT-001 Pro has a greater chance of success. The total U.S. potential market size for the drug is $550 million, with an enhanced version of Imatinib (IkT-001 Pro); if the company is able to capture even 12% of the market, the potential sales could be $65 million.
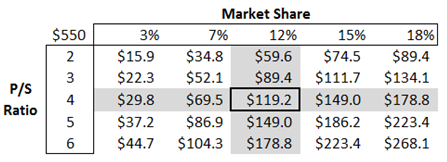
In the above exhibit, it has been assumed that the company will get approval for IkT-0001 Pro, and the company will commercialize the drug in the next four years. An implied market cap has been calculated based on a 12.0% market share, and the price to sales of 4.0x, when discounted at a 22.0% discount rate for four years, comes out to be $119.20 million or $12.0/share.
If the company gets the approval for IkT-148009, which is the company’s lead small drug candidate, and any progress is seen in other drug candidates, the shareholder value creation will be significantly greater than the projection mentioned above.
