HLA Typing For HSCT: New Research Indicates Expanded Patient Compatibility
By Selena Yu, GlobalData
Human leukocyte antigen (HLA) typing is a molecular diagnostic tool important for understanding immune compatibility, which is used in hematopoietic stem cell transplantation (HSCT). HSCTs can occur with bone marrow, peripheral blood stem cells (PBSC), or umbilical cord blood. Ninety percent of HSCTs use PBSCs because it is a more convenient, practical, and safer option. The HLA loci, encoded by a cluster of highly polymorphic genes on chromosome 6, support antigen presentation and immune recognition. Typically, in HLA typing, -A, -B, -C, -DRB1, -DQB1, and -DPB1 loci are tested. Accurate HLA typing is indispensable in transplantation immunology, where it enables precise matching of donors and recipients, thus minimizing the risk of allograft rejection and improving patient outcomes. Beyond transplantation, HLA typing is integral to understanding the genetic basis of autoimmune diseases, hypersensitivity reactions, and disease associations such as celiac disease, ankylosing spondylitis, and rheumatoid arthritis.
The primary challenge is finding a fully matched (HLA-A, -B, -C, -DRB1) HSCT unrelated donor. Despite over 44 million unrelated donors being listed in registries as of 2023, 25%-80% of U.S. patients are unable to find an HLA-matched unrelated donor (MUD), with a significant gap affecting patients with ethnically-diverse backgrounds.1,2 Advances in next-generation sequencing (NGS) and type-matching research in HSCT have revolutionized transplant care, providing unparalleled accuracy and resolution to meet the demands of modern immunogenetics and clinical practice. It also raises the question of how these technical and clinical advancements will affect the HLA typing market.
Research In Mismatched Unrelated Donors
In the past decade, significant advances in HSCT research have allowed more patients to be matched, thus improving patient outcomes. A pivotal single-center study by Kasamon et al., published in 2017, showed that using mismatched unrelated donors (MMUD) via bone marrow transplants was a safe and practical possibility.3 Before this, the standard was first to look for a matched related donor (MRD) within the patient’s family. Since 70% of patients have an 8/8 match (an HLA-A, -B, -C, and -DRB1 match) in their family, 30% would need to find an MUD. However, a haploidentical (a sibling half-match) donation with a high dose of post transplantation cyclophosphamide (PTCy), to prevent rejection, had comparable results to MRD and MUD.3 This study aimed to look at mismatched unrelated donors that shared ≥5/10 but <10/10 HLA alleles coupled with PTCy, and it found that over a four-year median follow-up on 19 patients, the overall survival was 75% in the first year and 69% in the second year.3
This single-center study in MMUD sparked the 15-MMUD multi-center trial (NCT02793544), which looked at 80 HSCTs with MMUD at 4/8 to 7/8 HLA alleles and PTCy, sirolimus, and mycophenolate mofetil (MMF) graft-versus-host disease (GVHD) prophylaxis using bone marrow.2 The study, sponsored by the National Marrow Donor Program (NMDP) and conducted by the Center for International Bone Marrow Transplant Research (CIBMTR), achieved a one-year overall survival of 72% myeloablative (MAC) and 79% in reduced-intensity conditioning (RIC) strata with 48% of enrollments being ethnic minorities and 39% of pairs being 4 to 6 out of 8 HLA allele matches.2 A few years later, the three-year survival was tested, and it was found that the three-year outcome was 62% and 70% in the MAC and RIC strata, respectively.4
The 15-MMUD trial outcomes show the clinical utility of using MMUD bone marrow at a 4 to 6 out of 8 HLA allele match to bridge the unmet need of HSCT donors in the U.S. Moreover, a larger retrospective study with 10,025 HSCT recipients, looking at MUD and MMUD treated with either calcineurin inhibitor (CNI) or PTCy, demonstrated that MUD and MMUD treated with PTCy had comparable outcomes, suggesting that MMUDs are a viable donor option when MUD is not available.5 Thus, it proved on a larger scale that using MMUD HSCT with PTCy can expand access to care to individuals, especially for ethnic and racial minorities.
Following the success of the 15-MMUD, NMDP sponsored another study, the ACCESS trial (NCT04904588), to analyze the utility of MMUDs using peripheral blood stem cells in adults with hematologic malignancies and bone marrow in pediatric patients.6 This study aims to evaluate the use of PBSCs as a graft source, given that PBSCs are a more common donation type, offer greater safety for donors, involve a less complex procedure, and provide more predictable product quality compared to bone marrow. Interim results released for the ACCESS trial in February 2025 showed that 84% of overall survival at one year exceeded the primary endpoint of 75% in the MAC cohort.7 In the RIC cohort, patients exhibited a 79% overall survival with a significant 51% GVHD-free, relapse-free survival, one-year post transplant.8 Lastly, NMDP and CIBMTR initiated the OPTIMIZE clinical trial (NCT06001385), which aims to be completed in 2026, to study the impact of using a reduced PTCy dose after a patient receives PBSCs from an MMUD on GVHD protection compared to the standard PTCy dose.9 This is vital as the standard dosage of PTCy for MMUD has been associated with an increased risk for bacterial and viral infections and delayed engraftment. The OPTIMIZE and ACCESS trials, sponsored by NMDP, will further improve MMUD HSCT patient care, coupling MMUD pairing, reducing PTCy usage, and expanding donation pools to historically underserved communities.
While major research has occurred in MMUD, the current donation landscape in the U.S. shows that the minority of transplants were MMUDs in 2022. According to the CIBMTR, in the U.S., from 2017 to 2022, there was a 66.7% increase in MMUD transplants.10 The majority, 45%, of allogeneic HSCTs in 2022 occurred with MUD; 20% of transplants occurred with haploidentical donors, with MRD at 21% and MMUD at 9%. As research in this field expands and more data supports MMUD, we can expect an increase in MMUD adoption in the future.
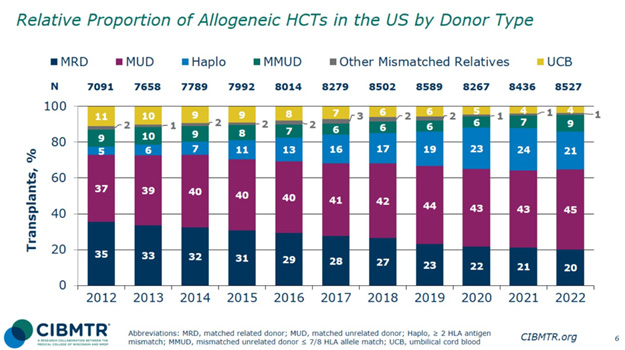
Figure 1: Relative Proportion of Allogeneic HCTs in the U.S. by Donor Type10
HSCT Donors For Ethnic And Racial Minorities
NMDP’s research is groundbreaking as it will open care for individuals, typically patients from ethnic and racial ancestries, to MMUDs in the current registry system. A prior registry modeling study analyzing over 10.5 million adult donors from the NMDP Registry found that patients with Black and South or Central American ancestry had a 16% likelihood of finding an MUD, whereas white patients had a 75% likelihood.11 When the NMDP reevaluated the registry modeling in 2023 to determine if MMUD can bridge the gap in finding a suitable donor, they found that by expanding unrelated donor matching from 7/8 to 5/8, the likelihood of finding a match increased to ≥99% for all racial groups, even when considering donors age ≤35 years and donors of all ages regardless of ancestry.1 Just expanding from a MUD to a 7/8 MMUD increases donor availability overall from 66% to 97%.1 Thus, by coupling the groundbreaking findings that MMUD, as low as 4/8, has similar patient survival when treated with PTCy compared to matched donations and that by just expanding mismatching donations to a 7/8 HLA allele, all patients in need can receive an HSCT.
The Importance Of NGS HLA Type Testing In MMUD
Bridging the gap in HSCT treatment requires high-resolution HLA typing. According to NMDP’s Senior Director in Vendor and Biorepository Services Jason Dehn, the NMDP registry in the U.S. has been using NGS for the past decade. NGS offers three advantages – high throughput, deeper sequencing information, and higher accuracy – compared to traditional high-resolution techniques like sequence-specific oligonucleotide probe (SSO) and sequence-based typing (SBT). NGS HLA typing tests can be conducted in large batches as the tests can be one plate that can run up to 96 samples at once and results are ready within 48 hours with high accuracy and deeper sequencing at a lower cost than the other techniques. With the increased matching of unrelated donors and patients over time in the U.S. and globally, we can expect an increase in NGS as unrelated donations typically require higher resolution testing compared to related donations. If this trend continues over the next 10 to 15 years, millions of individuals who previously could not find a matched donor may now have access to potential mismatched donors.12 Moreover, NGS HLA typing for all relevant loci can occur with one test, which can be more cost-effective for labs. Donors listed in international registries have HLA typing confirmed, typically using NGS methods, each time a new patient match is evaluated, prior to transplant. Lastly, the WMDA 2023 report found that, globally, only 45% of unrelated blood stem cell donors have been HLA typed for HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1 and 15% have been typed for HLA-A, -B, -C, -DRB1, and -DQB1.13 Thus, we may see an increase in NGS tests in the next five to 10 years with increased donor testing volumes to serve an expanded population of patients seeking HSCT due to the recent findings that MMUD HSCT patients have similar overall survival rates compared to MRD and MUD HSCTs.
Conclusion
Recent research conducted in the HSCT space, specifically in MMUD, has opened the door for millions of patients seeking treatment globally for blood cancers and diseases. Currently, the likelihood of finding a match for Black and South or Central American patients can be as low as 16% due to limited donors in those racial groups.10 Therefore, expanding unrelated HSCT donor criteria from a full 8/8 match to a 7/8 match increases donor availability by 31%, while expanding to a 5/8 match raises the likelihood of finding a donor to over 99%.1 Research sponsored by NMDP also has demonstrated that the overall survival of patients with MMUD is comparable to MUD and MRD, thus providing a safe alternative when matched donation is not available.2,4,5 By bridging this gap in health access with innovative and groundbreaking research, patients suffering from blood cancers like AML and MDS can find treatment. As more studies and positive results from MMUD HSCTs become available over the next decade, physicians and other healthcare professionals will see the value in mismatched transplants. Research and clinical validation are half the battle to improving patient care with MMUD; the other half lies with the healthcare professionals who inform patients of different types of allogenic HSCT and what is best suited for them. Thus, the expansion of HSCT access is a multifaceted conversation that includes more pivotal research to improve MMUD patients, educating HSCT-responsible bodies about the benefits of MMUD, and increasing ethnic diversity among donors. Additionally, NGS HLA testing will increase because of its high volume and accuracy and low cost compared to other high-resolution HLA tests. Advances in NGS and type-matching research in HSCT have transformed transplant care, delivering unmatched accuracy and resolution to address the evolving needs of patients.
References
- Chowdhury, Maiers, Spellman et al. "Existence of HLA-Mismatched Unrelated Donors Closes the Gap in Donor Availability Regardless of Recipient Ancestry," Transplantation and cellular therapy, p. 686.e1–686.e8., 2023.
- Shaw, Jimenez-Jimenez, Burns et al. "National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide.," Journal of clinical oncology : official journal of the American Society of Clinical Oncology, p. 1971–1982, 2021.
- Kasamon, Ambinder, Fuchs et al."Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide.," Blood Adv, p. 288–292, 2017.
- Shaw, Jimenez-Jimenez, Burns et al., "Three-Year Outcomes in Recipients of Mismatched Unrelated Bone Marrow Donor Transplants Using Post-Transplantation Cyclophosphamide: Follow-Up from a National Marrow Donor Program-Sponsored Prospective Clinical Trial.," Transplantation and cellular therapy, p. 208.e1–208, 2023.
- Shaffer, Gooptu, DeFor et al., "Post-Transplant Cyclophosphamide-Based Graft-Versus-Host Disease Prophylaxis Attenuates Disparity in Outcomes Between Use of Matched or Mismatched Unrelated Donors.," Journal of clinical oncology : official journal of the American Society of Clinical Oncology, p. 3277–3286, 2024.
- Center for International Blood and Marrow Transplant, "HLA-Mismatched Unrelated Donor Hematopoietic Cell Transplantation With Post-Transplantation Cyclophosphamide (ACCESS)," 2021. [Online]. Available: https://clinicaltrials.gov/study/NCT04904588.
- J. Ayers, "New, Affirming Hematopoietic Cell Transplant Results Highlight Approaches to Increase Patient Access at 2025 Tandem Meetings," NMDP , 15 February 2025. [Online]. Available: https://network.nmdp.org/news-events/newsroom/new-affirming-hematopoietic-cell-transplant-results-show-increase-patient-access-at-2025-tandem [Accessed 5 March 2025]
- Al Malki, Bo-Subait, Logan et al., "Post-transplant cyclophosphamide-based graft-versus-host disease prophylaxis following mismatched unrelated donor peripheral blood stem cell (PBSC) transplantation.," Journal of Clinical Oncology, 2024.
- Center for International Blood and Marrow Transplant, "HLA-Mismatched Unrelated Donor Peripheral Blood Stem Cell Transplantation with Reduced Dose Post Transplantation Cyclophosphamide GvHD Prophylaxis (OPTIMIZE)," 2023. [Online]. Available: https://clinicaltrials.gov/study/NCT06001385?term=NCT06001385&rank=1.
- Cusatis, Litovich, Feng et al., "Current trends and outcomes in cellular therapy activity in the United States, including prospective Patient Reported Outcomes data collection within the CIBMTR registry," Transplantation and cellular therapy, p. 917.e1–917.e12, 2024.
- Gragert, Eapen, Williams et al., "HLA match likelihoods for hematopoietic stem-cell grafts in the US registry," New England Journal of Medicine,, pp. 339-348., 2014.
- Niederwieser, Baldomero, Bazuaye et al., "One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors.," Haematologica, 2022.
- WMDA, "WMDA Global Trends Report 2023," 2023.
About The Author:
 Selena Yu is a senior medical devices analyst at GlobalData, with a focus in diagnostics. Before joining GlobalData, Yu earned MSc and BSc degrees in microbiology and immunology from McGill University, where her work focused on finding therapeutic and vaccine candidates for the neglected tropical disease schistosomiasis.
Selena Yu is a senior medical devices analyst at GlobalData, with a focus in diagnostics. Before joining GlobalData, Yu earned MSc and BSc degrees in microbiology and immunology from McGill University, where her work focused on finding therapeutic and vaccine candidates for the neglected tropical disease schistosomiasis.
