A surprise mimicker of a rare disorder: 13-year-old female with altered mental status
13-year-old female with altered mental status, headache and emesis. What is the diagnosis?
Presentation
A 13-year-old female with a history of iron deficiency anemia presented with altered mental status preceded by 5 days of headache, vomiting, nausea, and worsening somnolence that was not responsive to flumazenil, naloxone, or ammonia salts. She had a Glasgow coma scale of 12 and was drowsy with no cranial nerve deficits, or other abnormal physical examination findings. Laboratory evaluation showed microcytic anemia (hemoglobin 7.7 g/dL and MCV [mean corpuscular volume]:64.7, lymphocytosis, and mildly elevated liver enzymes [Aspartate Aminotransferase [AST] 52, Alanine Transaminase [ALT] 110) otherwise her electrolytes and kidney function were normal.
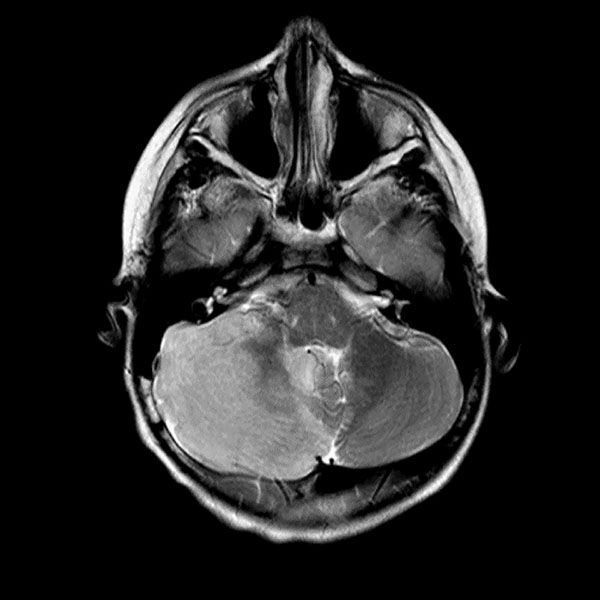
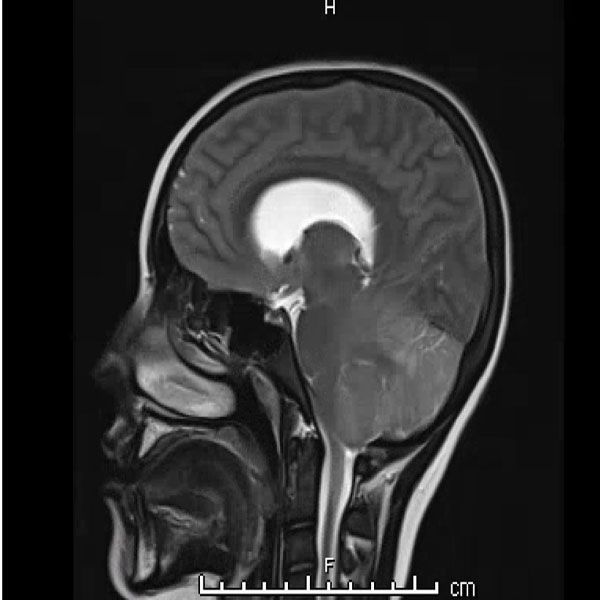
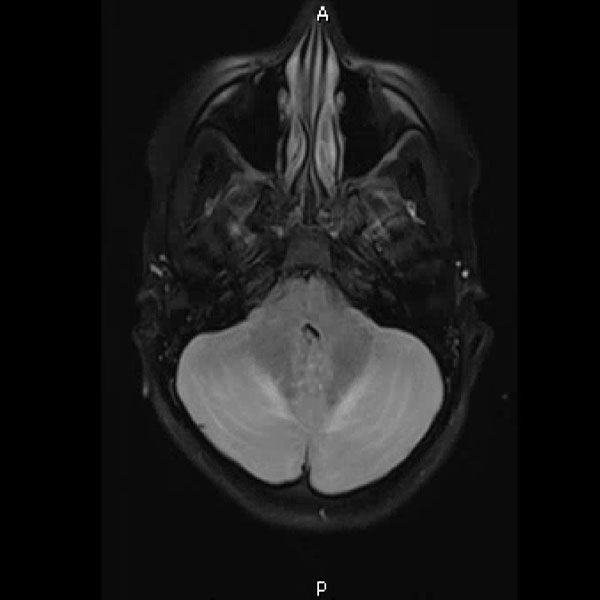
Urine toxicology, Tylenol level, Salicylate level, alcohol level thyroid studies, iron studies, hepatitis panel were normal. Urine pregnancy and COVID tests were negative. Head computed tomography (Head CT) showed mild hydrocephalus, and brain magnetic resonance imaging (MRI) showed increased signal folia and compression of the lower portion of the fourth ventricle and foramen of Magendie. She then had an emergency external ventricular drain placed (EVD) by pediatric neurosurgery, and the team started her on high-dose steroids and intravenous immunoglobulin (IVIG).
On the third day of admission, she developed new onset left-sided facial drooping, drooling, downbeat nystagmus, and difficulty following commands. An emergent subcortical craniectomy and decompression, duraplasty, and cerebellar biopsy were performed. A cerebellar biopsy was performed due to findings on brain MRI.
Consults were placed with pediatric neurology, pediatric infectious disease, pediatric hematology. Etiologies considered, included infectious, heavy metal poisoning, autoimmune, metabolic, and oncologic, with the following studies ordered: Cytomegalovirus Antibody(Ab) panel, and DNA, Coccidioides Ab, Epstein Barr Virus titers, fungal cerebrospinal fluid(CSF) panel, Human Immunodeficiency Virus testing, mycoplasma pneumoniae Ab, tuberculosis testing, toxoplasma Ab, meningitis panel, lactate dehydrogenase , uric acid, blood smear, CSF cytology, heavy metal panel, lead level, hemoglobin electrophoresis, complete blood count, complete metabolic panel, anti-nuclear Ab, N-Methyl-D-Aspartate receptor Ab, and ammonia. Lhermitte-Duclos disease versus cerebritis were also considered based on clinical status and radiological findings on brain MRI (Table 1).
Final diagnosis
Subsequently, her lead level returned from the state lab, elevated at 99 mcg/dL, and in consultation with a medical toxicologist, she was started on succimer and dimercaprol.
Further discussion with the parents revealed that the patient had been chewing on imported traditional pottery and eating out of glazed metal pots. Serial lead levels were drawn during her stay. Her lead levels fell to as low as 41 mcg/dL but then, on a follow-up draw, increased to 54 mcg/dL several days later. The several subsequent draws would fluctuate between 55-61 mcg/dL. It was believed that these levels were due to lead leaching from her bones due to her chronic exposure.
Further investigation also revealed that she had been chewing on her nails while in the hospital after applying foreign-manufactured nail polish. She completed 4 weeks of succimer therapy and was placed on a 2-week drug holiday. At discharge, her lead level was 49 mcg/dL, and follow-up lead testing was to be done by her pediatrician. She had marked improvement in her neurological function but still had residual 6th nerve palsy and papilledema, which she needed to follow up with ophthalmology. She would also follow up with her primary care provider (PCP) for lead level and general post-discharge monitoring, neurosurgery to determine when she will have her cranioplasty, hematology for iron deficiency anemia, and cardiology for supraventricular tachycardia (SVT), which she had developed during her hospital stay. Multiple items in the home contained lead.
Her biopsy result had nonspecific findings not consistent with Lhermitte-Duclos disease which is an extremely rare tumor of the cerebellum (dysplastic cerebellar gangliocytoma). The final diagnosis was lead encephalopathy.
Discussion
Unfortunately, lead poisoning exists worldwide.1-4 It can present in several ways, from asymptomatic to severe neurological deficits mimicking rare conditions like Lhermitte-Duclos disease, as was the case in our patient. Myriad items have been implicated from contaminated water, such as in the Flint, Michigan water crises5, lead paint from old houses, leaded gasoline in developing world countries, air pollution, pottery, and spices.1-4,6-8
Any decision to start chelation therapy should be determined in consultation with medical toxicology. State inspectors were sent to investigate the patient's home for lead testing, where pottery, fingernail polish, and other items were removed from the home pending a final report before the patient was discharged.
The state report showed several items and areas of the household with high lead levels. Samples with elevated levels were anise seeds (0.144 ppm), cinnamon sticks (0.438 ppm), Hibiscus/Jamaica (0.084 ppm), lemongrass tea (0.065 ppm), marjoram (0.350 ppm), glazed clay pieces (0.101 ppm), blue nail polish (0.207 ppm), and pink nail polish (0.380 ppm). There is no safe lead level and unfortunately, lead exposure and lead poisoning are not in the archives.2
As was the case in our patient, there is continued exposure to lead from imported traditional pottery.9 The CDC reported in November 2010 that there was certain imported traditional pottery labeled as lead-free which contained extractable amounts of lead comparable to lead-glazed pottery. Brightly colored orange, red or yellow pottery most often is lead which is used to enhance to increase the intensity of these pigments.9 Even when the non-lead glaze has been used in the manufacturing process, lead could still be on the surfaces of this pottery due to firing in ovens containing lead from previous use with lead-based glaze.9

Lead Prevention would involve primary and secondary interventions, such as removing lead-containing items in the environment before exposure and lead level testing by primary care providers, follow-up, and referral, respectively.10
Lead exposure poisoning could be asymptomatic but lead to learning disabilities, low IQ, and inattention.10 The current blood lead level threshold or reference value used by the CDC is 3.5 micrograms per dl.10
Lead testing is usually recommended for children at 12 months and 24 months with a capillary blood sample and if blood levels are greater than 3.5 micrograms per dl then a venous stick is performed.10,11
Below is a table from the CDC showing recommended blood testing intervals for various blood lead levels.10
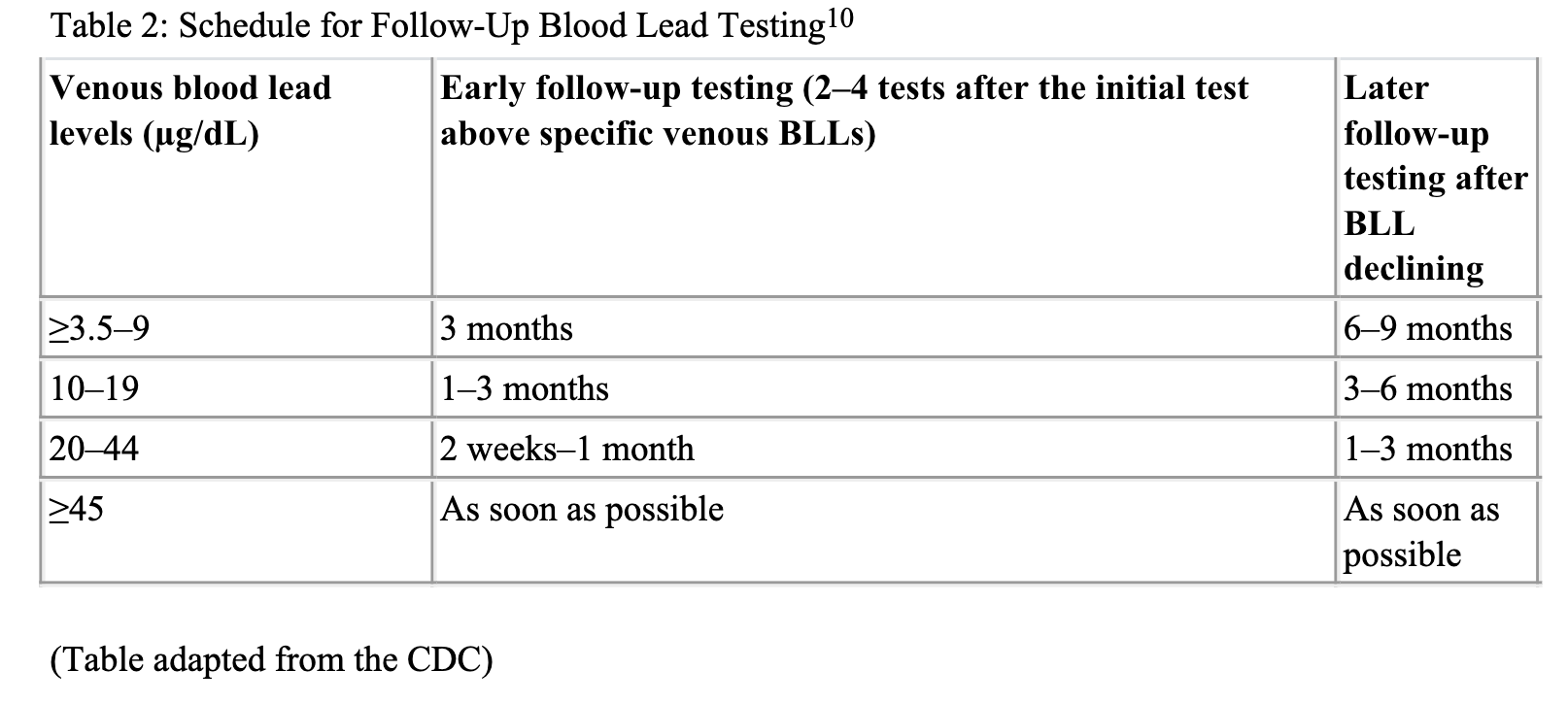
Children with elevated blood lead levels should also be screened for Iron deficiency anemia, calcium, magnesium, and zinc deficiency since these elements co-share the same transporter system. In the low levels of the respective mentioned minerals, lead absorption increased.11
Lead poisoning management depends on the level and would include good environmental and nutritional history, removal of the offending lead-containing agent, nutritional counseling and treatment of deficiencies such as iron deficiency anemia, abdominal decontamination, laboratory testing such as complete blood count, electrolytes, blood urea nitrogen, creatinine, liver transaminase enzyme levels, and urinalysis, and chelation therapy.11
Chelation therapy should be done with a medical toxicologist and commonly used chelation agents include Succimer, Dimercaprol, and CaEDTA.
Follow-up
After discharge, she continued to improve. She did not require outpatient physical therapy, and she was homebound until after her cranioplasty 7 months later.
On her last visit with her PCP, she still had difficulties catching up with schoolwork, which she attributed to the many lost school days. She continues to take iron for her iron deficiency anemia, and her 6th nerve palsy, papilledema, and SVT have since resolved. Of note, her lead levels continued to remain elevated (69-79-77-75-81-70-59-60.3). This was discussed with the medical toxicologist several times, and chelation was not recommended.
The elevated levels were attributed to leaching from bone, which could continue for many years. As of this writing, the last level was 60.3mcg, and she continues to do well with no repeat acute decompensation. Blood lead level usually decreases after chelation therapy but could rebound within days to weeks, as in our patient.
Chelation therapy should not be commenced as an outpatient if the lead source has not been removed or abated. If multiple courses of chelation therapy are administered, it will be imperative to monitor levels of essential trace elements.12
Lessons learned
Her case highlights that lead poisoning can present itself in many ways and, in its severe form, encephalopathy, which could even mimic rare conditions such as Lhermitte-Duclos disease.
It also illustrates the variety of items that can be contaminated with lead and cause poisoning, and therefore, high index suspicion and good history should be made to determine exposure to foreign or imported spices, cosmetics, and pottery or cooking utensils.1,3,4,7
This case also highlights the need always to have a broad differential when the clinical picture is unusual or does not fit one's initial impression.
Unfortunately, lead poisoning is still a worldwide public health crisis, and the hope is that policies can be implemented to protect children and the public. Therefore, pediatricians should not be afraid to advocate for the safety of their patients and children at large.1,2,4,5,7.
This case is based on a presentation by Drs (Amanda Purser [previously Amanda Huggett], Cheyenne Bownds, Christian Molony, and Ngozi Eboh) at the (Pediatric Hospital Medicine, E-Poster session, Orlando, Florida), Poster Session: E-Poster, Presentation Date: July 29th, Poster Number: 1217672.
References:
- Angelon-Gaetz KA, Segule MN, Wilson M. Lead Levels in Spices From Market Basket and Home Lead Investigation Samples in North Carolina. Public Health Rep. 2023;138(1):91-96. doi:10.1177/00333549211066152
- Emond AM. Lead poisoning cannot be consigned to history books yet: new guidance to help us to reach that goal. Arch Dis Child. 2022;107(4):313-314. doi:10.1136/archdischild-2019-318756
- Hoang TG, Tran QP, Lo VT, Doan NH, Nguyen TH, Pham MK. Blood Lead Levels and Associated Sociodemographic Factors among Children Aged 3 to 14 Years Living near Zinc and Lead Mines in Two Provinces in Vietnam. Biomed Res Int. 2021;2021:5597867. Published 2021 Jul 6. doi:10.1155/2021/5597867
- Hore P, Alex-Oni K, Bardhi N, Sedlar S. Notes from the Field: Lead Poisoning in a Family of Five Resulting from Use of Traditional Glazed Ceramic Ware - New York City, 2017-2022. MMWR Morb Mortal Wkly Rep. 2022;71(22):743-744. Published 2022 Jun 3. doi:10.15585/mmwr.mm7122a3
- Abbasi J. Lead, Mistrust, and Trauma-Whistleblowing Pediatrician Discusses the Legacy of Flint's Water Crisis. JAMA. 2021;325(21):2136-2139. doi:10.1001/jama.2021.2314
- Jallad R, Rao MS, Rahman A. Prevalence of lead toxicity in adolescents in Kuwait. BMC Public Health. 2021;21(1):1189. Published 2021 Jun 22. doi:10.1186/s12889-021-11210-z
- Kappel M, Kraushaar V, Mehretu A, Slater W, Marquez E. Notes from the Field: Childhood Lead Poisoning Associated with Turmeric Spices - Las Vegas, 2019. MMWR Morb Mortal Wkly Rep. 2021;70(45):1584-1585. Published 2021 Nov 12. doi:10.15585/mmwr.mm7045a4
- Swaringen BF, Gawlik E, Kamenov GD, McTigue NE, Cornwell DA, Bonzongo JJ. Children's exposure to environmental lead: A review of potential sources, blood levels, and methods used to reduce exposure. Environ Res. 2022;204(Pt B):112025. doi:10.1016/j.envres.2021.112025
- FDA. Questions and answers on Lead-Glazed Traditional Pottery. FDA. October 27, 2017. https://www.fda.gov/food/environmental-contaminants-food/questions-and-answers-lead-glazed-traditional-pottery
- CDC. Preventing Childhood Lead Poisoning. CDC. June 12, 2024. https://www.cdc.gov/lead-prevention/prevention/index.html
- Pediatric Environmental Health Specialty Units. Recommendation on Management of Childhood Lead Exposure. Pediatric Environmental Health Specialty Units.Updated September 2021. https://www.pehsu.net/lib_facts/pehsu_fact_sheet_lead_management_for_health_professionals.pdf
- Markowitz M. Lead poisoning. Pediatr Rev. 2000;21(10):327-335. doi:10.1542/pir.21-10-327
Acknowledgment
Amanda Purser, DO, former resident (Class of 2023), Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, for her contribution to the initial draft of this case and presentation at pediatric hospital medicine, 2022.
Christian B. Molony, MD; former resident (Class of 2023), Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, for his contribution to the initial draft of this case
Cheyene. N. Bownds, MD; former resident (Class of 2023), Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, for his contribution to the initial draft of this case
Elizabeth Sledge, MD, former faculty, Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, for assisting with obtaining final consent for publication from the patient and family.
















