Practice Essentials
Human immunodeficiency virus (HIV) is a blood-borne virus typically transmitted via sexual intercourse, shared intravenous drug paraphernalia, and during the birth process or via human milk (vertical transmission). HIV disease is caused by infection with HIV-1 or HIV-2, which are retroviruses in the Retroviridae family, Lentivirus genus. [1, 2]
 Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
Signs and symptoms
The patient with HIV may present with signs and symptoms of any of the stages of HIV infection. No physical findings are specific to HIV infection; the physical findings are those of the presenting infection or illness. Manifestations include the following [1, 2] :
-
Acute seroconversion manifests as a flulike illness consisting of fever, malaise, and a generalized rash
-
The asymptomatic phase generally is benign
-
Generalized lymphadenopathy is common and may be a presenting symptom
-
AIDS manifests as recurrent, severe, and occasionally life-threatening infections or opportunistic malignancies
-
HIV infection can cause some sequelae, including AIDS-associated dementia/encephalopathy and HIV wasting syndrome (chronic diarrhea and weight loss with no identifiable cause)
The history should address risk factors for possible exposure to HIV, including the following:
-
Unprotected sexual intercourse, especially receptive anal intercourse
-
A large number of sexual partners
-
Previous or current sexually transmitted diseases (STDs)
-
Sharing of intravenous (IV) drug paraphernalia
-
Receipt of blood products (before 1985 in the United States)
-
Mucosal contact with infected blood or needle-stick injuries
-
Maternal HIV infection (for newborns, infants, and children)
See Clinical Presentation for more detail.
Diagnosis
HIV screening recommendations include the following:
-
The US Preventive Services Task Force (USPSTF) recommends that clinicians screen for HIV in all adolescents and adults at increased risk for HIV infection, and all pregnant individuals [3]
-
The Centers for Disease Control and Prevention (CDC) recommends opt-out HIV screening for patients in all healthcare settings; persons at high risk for HIV infection should be screened at least annually [4]
-
The American College of Physicians (ACP) recommends that clinicians adopt routine screening for HIV and encourage all patients to be tested [5]
-
CDC guidelines recommend testing for HIV infection with a US Food and Drug Administration (FDA)–approved antigen/antibody immunoassay that detects HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen, with supplemental testing after a reactive assay result to differentiate between HIV-1 and HIV-2 antibodies. If supplemental testing for HIV-1/HIV-2 antibodies shows nonreactive or indeterminate results (or if acute HIV infection or recent exposure is suspected or reported), an HIV-1 nucleic acid test is recommended to differentiate acute HIV-1 infection from a false-positive test result. [6]
-
The World Health Organization (WHO) recommends that all HIV testing algorithms achieve at least a 99% positive predictive value and use a combination of tests with greater than or equal to 99% sensitivity and greater than or equal to 98% specificity. The first test in an HIV testing strategy and algorithm should have the highest sensitivty, followed by a second and third test of the highest specificity. [7]
The CD4 T-cell count reliably reflects the current risk of acquiring opportunistic infections, as follows [1, 2] :
-
Reference range, 500-2000 cells/μL
-
Because CD4 counts vary, serial counts generally are a better measure of significant changes
-
After seroconversion, CD4 counts tend to decrease (~700/μL) and continue to decline over time
-
For surveillance, a CD4 count below 200/μL is considered AIDS-defining in the United States
-
In children younger than 5 years, the CD4 T-cell percentage is considered more important than the absolute count (< 25% is considered to warrant therapy)
-
In adults with chronic hepatitis C and low absolute CD4 T-cells, the CD4 percentage may also be more useful [8]
Viral load in peripheral blood is used as a surrogate marker of viral replication rate; however, quantitative viral-load assays should not be used as a diagnostic tool. Clinical relevance is as follows:
-
Rate of progression to AIDS and death is related to the viral load; patients with viral loads greater than 30,000/mL are 18.5 times more likely to die of AIDS than those with undetectable viral loads.
-
With therapy, viral loads often can be suppressed to an undetectable level (< 20-75 copies/mL; optimal viral suppression); complete inhibition of viral replication appears impossible and may be unnecessary
-
Successfully treated patients may demonstrate intermittent low-level viremia (eg, < 400 copies/mL), but this is not thought to represent viral replication or to predict virologic failure (defined as a confirmed viral load of > 200 copies/mL [9]
In August 2013, the FDA approved Alere Determine HIV-1/2 Ag/Ab Combo test (Orgenics, Ltd) as the first rapid HIV test for the simultaneous detection of HIV-1 p24 antigen as well as antibodies to both HIV-1 and HIV-2 in human serum, plasma, and venous or fingerstick whole blood specimens. [10, 11] The test does not distinguish between antibodies to HIV-1 and HIV-2, and is not intended to be used for screening of blood donors. [10, 11]
Baseline studies for other infections that are important in the initial workup of a patient with newly diagnosed HIV infection include the following [1, 2] :
-
Purified protein derivative (PPD) skin testing for tuberculosis or interferon-gamma release assay
-
Cytomegalovirus (CMV) testing
-
Syphilis testing
-
Rapid amplification testing for gonococcal and chlamydial infection
-
Hepatitis A, B, and C serology
-
Anti- Toxoplasma antibody
-
Ophthalmologic examination
The CDC classifies HIV infection into 3 categories, as follows [12] :
-
Category A: Asymptomatic HIV infection without a history of symptoms or AIDS-defining conditions
-
Category B: HIV infection with symptoms that are directly attributable to HIV infection (or a defect in T-cell–mediated immunity) or that are complicated by HIV infection
-
Category C: HIV infection with AIDS-defining opportunistic infections
These 3 categories are further subdivided on the basis of the CD4 T-cell count, as follows:
-
> 500/µL: Categories A1, B1, C1
-
200-400/µL: Categories A2, B2, C2
-
< 200/µL: Categories A3, B3, C3
See Workup for more detail.
Management
Department of Health and Human Services (DHHS) guidelines on the timing of initiation of antiretroviral therapy are as follows [13] :
-
Antiretroviral therapy (ART) is recommended in all persons with HIV infection to reduce morbidity and mortality and to prevent HIV transmission to others.
-
The Panel on Antiretroviral Guidance for Adults and Adolescents recommends initiating ART immediately (or as soon as possible) after diagnosis to increase the uptake of ART linkage to care and to hasten and improve the rate of viral suppression.
-
When initiating ART, it is important to educate patients regarding the benefits of ART and to deploy strategies to optimize care engagement and treatment adherence.
-
Initiating ART is particularly important in patients with AIDS-defining conditions, patients with acute or recent HIV infection, and pregnant patients. Delaying therapy in these subpopulations has been associated with high risks for morbidity and mortality and HIV transmission.
-
Durable viral suppression improves immune function and overall quality of life, lowers the risk for both AIDS-defining and non–AIDS-defining complications, and allows persons with HIV infection to live a lifespan approaching that of persons without HIV infection. Two large randomized controlled trials, ART-START and TEMPRANO, demonstrated reductions in morbidity and mortality among individuals with HIV infection who had CD4 T-lymphocyte (CD4) cell counts of greater than 500 cells/uL and who were randomized to receive ART immediately compared with individuals in whom ART initiation was delayed.
-
All persons with HIV infection should be informed that maintaining a plasma HIV RNA (viral load) of less than 200 copies/mL with ART prevents sexual transmission of HIV to their partners. Patients may recognize this concept as “Undetectable = Untransmittable (U=U).” For persons with HIV infection who intend to rely on treatment as prevention (TasP), providers should make an individual assessment of the person's risk tolerance, personal health, history of maintaining viral suppression with treatment, and access to healthcare services and ART, as well as other factors that may affect their ability to maintain a high level of adherence to ART. Concerns regarding immune reconstitution inflammatory syndrome (IRIS): For some OIs, such as cryptococcal and TB meningitis, immediate ART initiation may increase the risk for serious IRIS. A short delay before initiating ART may be warranted. [14, 15, 16, 17] After ART initation, the patient should be closely monitored for signs and symptoms associated with IRIS.
Highly active antiretroviral therapy (HAART) is the principal method for preventing immune deterioration. Classes of antiretroviral agents include the following:
-
Nucleoside reverse transcriptase inhibitors (NRTIs)
-
Protease inhibitors (PIs)
-
Nonnucleoside reverse transcriptase inhibitors (NNRTIs)
-
Fusion inhibitors
-
CCR5 co-receptor antagonists (entry inhibitors)
-
HIV integrase strand transfer inhibitors
-
Entry inhibitors (CD4-directed post-attachment inhibitors)
-
Capsid inhibitors
DHHS guidelines list the following regimens as preferred in most treatment-naive patients [13] :
-
An initial antiretroviral (ARV) regimen for a treatment-naive patient generally consists of two nucleoside reverse transcriptase inhibitors (NRTIs) administered in combination with a third active ARV drug from one of three drug classes: an integrase strand transfer inhibitor (INSTI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a protease inhibitor (PI) with a pharmacokinetic (PK) enhancer (also known as a booster; the two drugs used for this purpose are cobicistat and ritonavir).
-
Data also support the use of the two-drug regimen, dolutegravir plus lamivudine, for initial treatment.
-
Before initiating antiretroviral therapy (ART) in a person of childbearing potential, clinicians should discuss the person’s intentions regarding pregnancy, and a pregnancy test should be performed (AIII). Clinicians should refer to the Perinatal Guidelines for recommendations on initial ARV regimen for an ART-naive person around the time of conception and during pregnancy.
The Panel on Antiretroviral Guidelines for Adults and Adolescents (the Panel) classifies the following regimens as Recommended Initial Regimens for Most People with HIV (in alphabetical order):
For those with HIV who have no prior use of long-acting cabotegravir (CAB-LA) as pre-exposure prophylaxis (PrEP), the following regimens are recommended:
-
Bictegravir/tenofovir alafenamide/emtricitabine (AI) (There are insufficient data to recommend Bictegravir during pregnancy.)
-
Dolutegravir/abacavir/lamivudine—only for individuals who are HLA-B*5701 negative and without chronic hepatitis B virus (HBV) coinfection (AI)
-
Dolutegravir plus (emtricitabine or lamivudine) plus (tenofovir alafenamide [TAF] or tenofovir disoproxil fumarate [TDF]) (AI)
-
Dolutegravir/lamivudine (AI)—except for individuals with HIV RNA >500,000 copies/mL, HBV coinfection, or when ART is to be started before the results of HIV genotypic resistance testing for reverse transcriptase or HBV testing are available.
For those with HIV and prior use of CAB-LA as PrEP, INSTI genotypic resistance testing should be done before beginning ART. If treatment is begun before receiving results of genotypic testing, the following regimen is recommended:
-
Boosted darunavir plus (TAF or TDF) plus (FTC or 3TC)—pending the results of the genotype test (AIII).
DHHS guidelines further recommend the following:
-
To address individual patient characteristics and needs, the Panel also provides a list of Recommended Initial Regimens in Certain Clinical Situations (see Table 6 below).
-
Given the many excellent options for initial therapy, selection of a regimen for a particular patient should be guided by such factors as virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance test results, comorbid conditions, access, and cost. Table 7 provides guidance on choosing an ARV regimen based on selected clinical case scenarios. Table 9 highlights the advantages and disadvantages of different components in a regimen.
-
Patients without prior ART who wish to begin long-acting intramuscular cabotegravir (CAB) and rilpivirine (RPV) should first achieve viral suppression on another regimen before shifting to oral, and then injectable, CAB and RPV (see Optimizing Antiretroviral Therapy in the Setting of Virologic Suppression).
ART should be initiated with one of the combination regimens recommended for persons with chronic HIV infection (AIII) (see What to Start). Providers should inform individuals starting ART of the importance of adherence to achieve and maintain viral suppression (AII). If available, the results of ARV drug resistance testing or the resistance pattern of the source person’s virus should be used to guide selection of the regimen. All persons of child-bearing potential should have a pregnancy test before initiating ART (AIII).
If ART is to be initiated before the results of drug resistance and HLA-B*5701 testing are available, one of the following regimens are appropriate options (AIII):
-
DTG with (emtricitabine [FTC] or lamivudine [3TC]) plus (tenofovir disoproxil fumarate [TDF] or tenofovir alafenamide [TAF])
-
BIC/TAF/FTC
-
Boosted darunavir (DRV) with (FTC or 3TC) plus (TAF or TDF)
DTG is a good treatment option because transmission of DTG-resistant HIV is rare, and DTG has a higher barrier to resistance than raltegravir and elvitegravir. Based on data from in vitro studies and clinical trials in ART-naive participants, it is anticipated that BIC, like DTG, also has a high barrier to resistance. However, clinical data and experience defining the BIC barrier to resistance are relatively limited at this time.
Preliminary data from Botswana suggested that there is an increased risk for neural tube defects (NTDs) (0.9%) in infants born to women who were receiving DTG at the time of conception. [18] Follow-up data, however, showed that the prevalence of NTDs in association with DTG exposure at conception is lower (0.3%), but still slightly higher than with non-DTG containing ARV regimens (0.1%). [19, 20] Before initiating an INSTI-based regimen in a person of childbearing potential, clinicians should review Table 6 for information to consider when choosing an ART regimen.
A pharmacologically boosted protease inhibitor (PI)-based regimen (eg, boosted DRV) is also an option, as resistance to PIs emerges slowly, and clinically significant transmitted resistance to PIs is uncommon. Abacavir/3TC is not recommended as part of an empiric treatment of acute HIV infection unless the patient is known to be HLA-B*5701 negative—information that seldom is available when individuals with acute infection present for care. Therefore, TDF/FTC or TAF/FTC generally is recommended as a backbone in this setting. Baseline laboratory testing recommended for individuals with chronic HIV infection should be performed (see Laboratory Testing for Initial Assessment and Monitoring of Patients with HIV Receiving Antiretroviral Therapy). Individuals with HBV/HIV coinfection should remain on TDF/FTC or TAF/FTC as part of their ART regimen.
Given the increasing use of TDF/FTC as pre-exposure prophylaxis (PrEP) in individuals who are HIV negative, [21, 22, 23] early infection may be diagnosed in some persons while they are taking TDF/FTC for PrEP. In this setting, drug resistance results are particularly important; however, the regimens listed above remain as reasonable treatment options pending resistance testing results.
INSTI-based regimens are as follows:
-
Bictegravir/tenofovir alafenamide/emtricitabine (single-tablet regimen)
-
Dolutegravir/abacavir/lamivudine (single-tablet regimen) - Only for patients who are HLA-B*5701–negative and without chronic hepatitis B virus (HBV) coinfection (In women of childbearing age, discuss the risks and benefits of prescribing dolutegravir around the time of conception, including the low risk for neural tube defects [NTDs] and the relative lack of information regarding the safety of using other commonly prescribed antiretrovirals [ARVs].)
-
Dolutegravir plus (emtricitabine or lamivudine) plus (tenofovir alafenamide or tenofovir disoproxil fumarate)
-
Raltegravir plus (emtricitabine or lamivudine) plus (tenofovir alafenamide or tenofovir disoproxil fumarate)
-
Dolutegravir plus lamivudine - Except in individuals with HIV RNA of more than 500,000 copies/mL, persons with HBV coinfection, or patients in whom ART is to be started before the results of HIV genotypic resistance for reverse transcriptase or HBV testing are available
The PI/r–based regimen is darunavir/ritonavir plus (tenofovir alafenamide or tenofovir disoproxil fumarate) plus (emtricitabine or lamivudine).
HIV-2 is intrinsically resistant to NNRTIs and enfuvirtide.
To address individual patient characteristics and needs, the Panel also provides a list of Recommended Initial Regimens in Certain Clinical Situations. [13]
Regimen selection is individualized based on the following:
-
Virologic efficacy
-
Toxicity
-
Pill burden
-
Dosing frequency
-
Drug-drug interaction potential
-
Drug resistance testing results
-
Comorbid conditions
-
Pregnancy
In particular cases, prophylaxis is indicated for specific opportunistic infections, including the following:
-
Pneumocystis jiroveci
-
Toxoplasma
-
Mycobacterium avium complex
-
Fungal and viral infections: Although prophylaxis for these infections is not routinely necessary, some recommend fluconazole in patients with CD4 T-cell counts under 50/µL to protect against cryptococcosis and endemic fungal infections. Oral fluconazole is not recommended for routine primary prophylaxis against Candida infection. Administration of ART and immune restoration are effective for preventing disease. Oral valganciclovir primary prophylaxis for CMV infection is not recommended in patients who will be receiving or are not receiving ART. [24]
Additional treatment measures include the following:
-
Treatment of opportunistic infections (directed at the specific pathogen)
-
Treatment of HIV lipodystrophy (tesamorelin)
-
Suppressive therapy for herpes simplex virus 2 (HSV-2) infection (acyclovir)
-
Treatment of HIV-associated diarrhea (crofelemer [25] )
The CDC has recommended HIV postexposure prophylaxis (PEP) and HIV pre-exposure prophylaxis (PrEP) regimens. [24]
PEP drug regimens are as follows:
-
Preferred option 1: Dolutegravir plus tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; Truvada)
-
Preferred option 2: Raltegravir plus Truvada (TDF/FTC)
-
Alternative: Truvada (TDF/FTC ) plus darunavir (Prezista) plus ritonavir daily
PrEP drug regimens are as follows:
-
Preferred PrEP drug regimen: Truvada (TDF/FTC)
-
Alternative: Descovy (TAF/FTC)
See HIV Infection and AIDS Treatment & Management for more detail.
Background
Human immunodeficiency virus (HIV) is a blood-borne, sexually transmissible virus (see the image below.) The virus typically is transmitted via sexual intercourse, shared intravenous drug paraphernalia, and perinatally during the birth process or via human milk.
 Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
The most common route of infection varies from country to country and even among cities, reflecting the population in which HIV was introduced initially and local practices. Co-infection with other viruses that share similar routes of transmission, such as hepatitis B, hepatitis C, and human herpes virus 8 (HHV8; also known as Kaposi sarcoma herpes virus [KSHV]), is common.
Two distinct species of HIV (HIV-1 and HIV-2) have been identified, and each is composed of multiple subtypes, or clades. All clades of HIV-1 tend to cause similar disease, but the global distribution of the clades differs. This may have implications on any future vaccine, as the B clade, which is predominant in the developed world (where the large pharmaceutical companies are located), rarely is found in the developing countries that are more severely affected by the disease.
HIV-1 probably originated from one or more cross-species transfers from chimpanzees in central Africa. [26] HIV-2 is closely related to viruses that infect sooty mangabeys in western Africa. [27] Genetically, HIV-1 and HIV-2 are superficially similar, but each contains unique genes and its own distinct replication process.
HIV-2 carries a slightly lower risk for transmission, and HIV-2 infection tends to progress more slowly to acquired immune deficiency syndrome (AIDS). This may be due to a less-aggressive infection rather than a specific property of the virus itself. Persons infected with HIV-2 tend to have a lower viral load than people with HIV-1, [28, 29] and a greater viral load is associated with more rapid progression to AIDS in HIV-1 infections. [30, 31]
HIV-2 is rare in the developed world. Consequently, most of the research and vaccine and drug development has been (perhaps unfairly) focused on HIV-1.
For information on HIV infection in children, see Pediatric HIV.
Initial description and early spread
In the United States, HIV disease was first described in 1981 among two groups, one in San Francisco and the other in New York City. Numerous young homosexual men presented with opportunistic infections that, at the time, were typically associated with severe immune deficiency: Pneumocystis pneumonia (PCP) and aggressive Kaposi sarcoma. [32]
HIV itself was not identified for another 2 years. [33] During that time, various other causes were considered, including lifestyle factors, chronic drug abuse, and other infectious agents. [34] The HIV epidemic spread rapidly and silently in the absence of testing.
However, clear clinical implications arose before society became aware of the disease; for example, prior to the recognition of HIV, only one case of Pneumocystis pneumonia not clearly associated with immune suppression was diagnosed in the United States between January 1976 and June 1980. In 1981 alone, 42 similar diagnoses were made, and by December 1994, 127,626 cases of Pneumocystis pneumonia with HIV infection as the only identified cause of immune suppression had been reported to the Centers for Disease Control and Prevention (CDC). Also, Kaposi sarcoma is up to 30,000 times more likely to develop in persons with HIV infection than in immunocompetent persons.
The spread of HIV was retrospectively shown to follow the trucking routes across Africa from logging camps, and the bush-meat trade combined with aggressive logging and improved transportation in the mid-20th century may have allowed what was likely occasional cross-species transmission events to propagate across the country and, eventually, the globe. [35]
Stigma of HIV infection
A considerable amount of stigma has been attached to HIV infection, mostly because of the virus's association with sexual acquisition and the inference of sexual promiscuity. Consequences of this stigma have included discrimination and reluctance to be tested for HIV infection. The stigma of HIV infection also is associated with a fear of acquiring a rapidly fatal infection from relatively casual contact.
Such attitudes are inappropriate because HIV is poorly transmissible without sexual contact or blood contact. In addition, the expected survival is long in patients with HIV infection who are receiving treatment. HIV is not transmitted during casual contact and is readily inactivated by simple detergents. Much of the concern regarding HIV infection is due to the incurability of the infection and the relentless immune decline and eventual premature death in the vast majority of infected people.
AIDS denialism
A small but vocal minority of people, including some scientists, continue to argue that HIV does not exist, or does not cause AIDS, and that the HIV tests are unreliable or that the therapies are toxic. Such misinformation usually is based on a lack of understanding of the scientific literature, deliberate misrepresentation, or logical fallacies based on pseudoscientific arguments.
All of the arguments proposed by these dissenters have been addressed and rebutted in the scientific literature and public discussion and even tested and rejected in the legal system. Nevertheless, they persist, and such views can have extremely harmful effects on people who are exposed to HIV infection unnecessarily or who refuse treatment for their progressing infection.
Clinicians should be aware of these issues, should be able and willing to address misinformation, and should direct their patients to reliable sources of information.
Political denial and inaction likely have caused considerable damage. Several governments in countries with high HIV infection rates were slow to admit that they had an HIV epidemic, and at least one (South Africa) initially rejected that AIDS was even a problem, then that the disease was caused by HIV infection, and, most recently, that antiretroviral therapy was effective in treating HIV infection and preventing MTCT. Changes have now occurred but have been slow and have cost hundreds of thousands of lives.
A regularly updated reference for addressing AIDS denial and misinformation can be found at AIDSTruth.org.
The quest for understanding of HIV
Since the discovery of HIV and its link to AIDS, great strides have been made in understanding its biology and in developing effective treatments. The difficulty in dealing with HIV on a global scale is largely due to the fact that HIV infection is far more common in resource-poor countries.
In the developed world, antiretroviral therapy has greatly improved prognosis and increased survival rates. Public education programs have raised awareness such that testing and prevention of infection are more common. Both of these approaches are difficult in countries with undereducated or underfunded populations.
A thorough discussion of the history of AIDS and the biologic link between HIV and AIDS can be found in an article entitled " The relationship between the human immunodeficiency virus and the acquired immunodeficiency syndrome " at the National Institute of Allergy and Infectious Diseases website. The document originally was written in September 1995, before the advent of highly active antiretroviral therapy (HAART), which has significantly improved AIDS-free survival in persons infected with HIV. This version was updated March 2010.
Patient confidentiality
HIV-related health information typically is considered separate from other health information and may require separate consent to share or divulge.
Healthcare workers who are infected with HIV may be required to divulge their status to their employer or patients and may be restricted in the types of procedures they can perform.
Pathophysiology
HIV produces cellular immune deficiency characterized by the depletion of helper T lymphocytes (CD4 cells). The loss of CD4 cells results in the development of opportunistic infections and neoplastic processes.
Virology of HIV
HIV-1 and HIV-2 are retroviruses in the Retroviridae family, Lentivirus genus. They are enveloped, diploid, single-stranded, positive-sense RNA viruses with a DNA intermediate, which is an integrated viral genome (a provirus) that persists within the host-cell DNA.
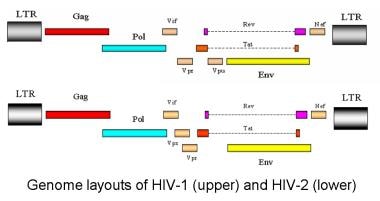
HIV contains three species-defining retroviral genes: gag, pol, and env. The gag gene encodes group-specific antigen; the inner structural proteins. The pol gene encodes polymerase; it also contains integrase and protease (the viral enzymes) and is produced as a C-terminal extension of the Gag protein). The env gene encodes the viral envelope—the outer structural proteins responsible for cell-type specificity. Glycoprotein 120, the viral-envelope protein, binds to the host CD4+ molecule.
HIV-1 has 6 additional accessory genes: tat, rev, nef, vif, vpu, and vpr. HIV-2 does not have vpu but instead has the unique gene vpx. The only other virus known to contain the vpu gene is simian immunodeficiency virus in chimpanzees (SIVcpz), which is the simian equivalent of HIV. [26] Interestingly, chimpanzees with active HIV-1 infection are resistant to disease. [36]
The accessory proteins of HIV-1 and HIV-2 are involved in viral replication and may play a role in the disease process. [37, 38] The outer part of the genome consists of long terminal repeats (LTRs) that contain sequences necessary for gene transcription and splicing, viral packaging of genomic RNA, and dimerization sequences to ensure that 2 RNA genomes are packaged.
The dimerization, packaging, and gene-transcription processes are intimately linked; disruption in one process often subsequently affects another. The LTRs exist only in the proviral DNA genome; the viral RNA genome contains only part of each LTR, and the complete LTRs are re-created during the reverse-transcription process prior to integration into the host DNA.
The biologic basis for AIDS
The specific details of the disease process that leads to AIDS are not fully understood despite considerable progress in the virology of HIV and the immunology of the human host, much of which has been driven by the urge to better understand AIDS. [39, 40, 41]
There is a specific decline in the CD4 helper T cells, resulting in inversion of the normal CD4/CD8 T-cell ratio and dysregulation of B-cell antibody production. [42, 43] Immune responses to certain antigens begin to decline, and the host fails to adequately respond to opportunistic infections and normally harmless commensal organisms. Because the defect preferentially affects cellular immunity, the infections tend to be nonbacterial (fungal, viral).
The pattern of opportunistic infections in a geographic region reflects the pathogens that are common in that area. For example, persons with AIDS in the United States tend to present with commensal organisms such as Pneumocystis and Candida species, homosexual men are more likely to develop Kaposi sarcoma because of co-infection with HHV8, and tuberculosis is common in developing countries.
Gut-associated lymphoid tissue (GALT) plays a role in HIV replication. [44] Although the portal of entry for HIV infection is typically through direct blood inoculation or exposure of the virus to genital mucosal surfaces, the GI tract contains a large amount of lymphoid tissue, making this an ideal site for HIV replication.
GALT has been shown to be a site of early viral seeding and establishment of the proviral reservoir. This reservoir contributes to the difficulty of controlling the infection, and efforts to reduce the levels of HIV provirus through sustained antiretroviral therapy (alone or in combination with interleukin-2 activation of resting HIV-infected T cells) have consistently failed. [45]
A feature of HIV replication in GALT is that it is compartmentalized, even among different areas of the gut. [46] Measurements of CD4 T cells in GALT show relatively less reconstitution with antiretroviral therapy than that observed in peripheral blood. [14, 15] At least one report has suggested that early treatment may result in better GALT CD4 T-cell recovery, [15] but clinical data generally argue against early initiation of therapy, which has not been shown to improve long-term survival.
In addition, HIV replication can be detected even in patients with supposedly suppressed replication, as judged by plasma viral load measurements. CD8+ killer T-cell responses to HIV occur in GALT and do not decline with antiviral therapy as much as peripheral measurements do. [16] These findings underscore the limitations of peripheral measurements in what is really a central viral replication.
One theory for the discrepancy between GALT and blood measurements is that ongoing viral replication in the lymphoid tissue, and the resulting immune activation, may actually hamper efficient CD4 T-cell replenishment. [17]
Studies of T-cell–replication kinetics have revealed that untreated HIV infection is characterized by rapid T-cell turnover but a defect in T-cell replication from the thymus. [47, 48, 49] These changes can be reversed with effective long-term antiviral therapy, [50, 51] suggesting that they are due to a direct effect of the virus or are a feature of the immune response against HIV.
It is known that normal cell cycling is necessary to produce a normal cytokine profile [52] and that HIV causes cell-cycle arrest. [18] Whether this is the exact mechanism is unresolved, however. Analysis of cytokine levels in HIV infected, uninfected, and HAART-treated patients with HIV show that cytokines involved in T-cell homeostasis were definitely affected, and therapy partially corrected these defects. In particular, there was decreased IL-7, IL-12, IL-15, and FGF-2, and increased TNF-alpha and IP-10. [19, 20]
Several of the HIV proteins directly affect T-cell function, either by disrupting cell cycling or down-regulating the CD4 molecule. The loss of T cells is clearly a primary issue, as the T-cell repertoire narrows in terms of which antigens the immune system will recognize and respond to. Antiviral therapy is able to reverse these changes, [21] but the degree of reversal is decreased if therapy is initiated very late in the infection and is further decreased when therapy is initiated when CD4 T-cell counts are 200/µL and below.
Direct cytotoxic effects of viral replication likely are not the primary cause of CD4 T-cell loss; a significant bystander effect [22] likely is secondary to T-cell apoptosis as part of immune hyperactivation in response to the chronic infection. Infected cells also may be affected by the immune attack.
One interesting issue is that the co-receptor usage of the virus strains tends to change over time. The initial infection nearly always involves a strain that uses the chemokine receptor 5 (CCR5), which is found on macrophages and dendritic cells, as a co-receptor with CD4. People who are homozygous for deletions in the CCR5 gene (ie, CCR5-delta32) tend to be resistant to infection, [23, 53] and those with heterozygosity for the polymorphism tend to show slower progression of disease. [54]
Over time, the receptor usage shifts to chemokine-related receptor (CXCR4) and other related receptors found on CD4 T cells. These virus strains are more likely to cause cell fusion (syncytia formation). This trend is far from absolute but does correlate in many people with disease progression. [55]
A single case report detailed a possible cure resulting from stem-cell transplantation from a CCR5-delta32 homozygous donor (performed to treat acute myelocytic leukemia). Although this important finding is unlikely to impact routine management of HIV infection, it does suggest that reconstitution of a host immune system with a population of mutant cells is a possible avenue of research to explore. [56]
Regardless of the cause for the disruption, a loss of thymic replacements in the face of an induced state of immune activation and T-cell loss seems to be a key component of the mechanism by which HIV narrows the T-cell repertoire and progresses to AIDS. [57, 58, 59]
Visible effects of HIV infection come in the form of disrupted lymph-node architecture. This disruption is temporal, and, at one point, lymph-node biopsy was considered as a form of staging the disease. [60, 61] The disruption of the follicular dendritic network in the lymph nodes and subsequent failure of normal antigen presentation are likely contributors to the disease process.
HIV replicates in activated T cells (its promotor contains a nuclear factor kappa B [NF-kappa-B]–binding region, the same protein that promotes other proteins in activated T cells and macrophages), and activated T cells migrate to the lymph nodes. As such, much of the viral replication occurs outside of the peripheral blood, even though serum viral load is still a useful surrogate marker of viral replication.
As mentioned above, with regards to GALT, HIV infection may be compartmentalized; specifically, areas of immune-privilege may occur such as in the testes and central nervous system where not only will there be differences in HIV pseudospecies but also different degrees of antiretroviral drug penetration. There is evidence that even with good peripheral control of HIV, the virus still may be detectable in the CSF and semen of some infected patients. [62, 63]
Phases of HIV infection
Clinical HIV infection undergoes 3 distinct phases: acute seroconversion, asymptomatic infection, and AIDS. Each is discussed below.
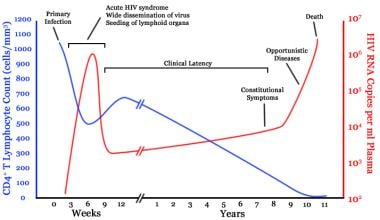 Timeline of CD4 T-cell and viral-load changes over time in untreated human immunodeficiency virus (HIV) infection. Courtesy of Wikipedia (based on an original from Pantaleo et al (1993)).
Timeline of CD4 T-cell and viral-load changes over time in untreated human immunodeficiency virus (HIV) infection. Courtesy of Wikipedia (based on an original from Pantaleo et al (1993)).
Acute seroconversion
Animal models show that Langerhans cells are the first cellular targets of HIV, which fuse with CD4 lymphocytes and spread into deeper tissues. In humans, rapid occurrence of plasma viremia with widespread dissemination of the virus is observed 4 days to 11 days after mucosal entrance of the virus.
There is no fixed site of integration, but the virus tends to integrate in areas of active transcription, probably because these areas have more open chromatin and more easily accessible DNA. [64, 65] This greatly complicates eradication of the virus by the host, as latent proviral genomes can persist without being detected by the immune system and cannot be targeted by antivirals.
During this phase, the infection is established and a proviral reservoir is created. [66, 67] This reservoir consists of persistently infected cells, typically macrophages, and appears to steadily release virus. Some of the viral release replenishes the reservoir, and some goes on to produce more active infection.
The proviral reservoir, as measured by DNA polymerase chain reaction (PCR), seems to be incredibly stable. Although it does decline with aggressive antiviral therapy, the half-life is such that eradication is not a viable expectation.
The size of the proviral reservoir correlates to the steady-state viral load and is inversely correlated to the anti-HIV CD8+ T-cell responses. Aggressive early treatment of acute infection lowers the proviral load, and treatment in newly infected (but postseroconversion) patients yields long-term benefit.
At this point, the viral load typically is very high, and the CD4 T-cell count drops precipitously. With the appearance of anti-HIV antibodies and CD8+ T-cell responses, the viral load drops to a steady state and the CD4 T-cell count returns to levels within the reference range, although slightly lower than before infection.
Seroconversion may take a few weeks, up to several months. Symptoms during this time may include fever, flulike illness, lymphadenopathy, and rash. These manifestations develop in approximately half of all people infected with HIV.
Asymptomatic HIV infection
At this stage in the infection, persons infected with HIV exhibit few or no signs or symptoms for a few years to a decade or more. Viral replication is clearly ongoing during this time, [68] and the immune response against the virus is effective and vigorous. In some patients, persistent generalized lymphadenopathy is an outward sign of infection. During this time, the viral load, if untreated, tends to persist at a relatively steady state, but the CD4 T-cell count steadily declines. This rate of decline is related to, but not easily predicted by, the steady-state viral load.
Evidence now shows that therapy initiation early in the asymptomatic period is effective. However, very late initiation is known to result in a less effective response to therapy and a lower level of immune reconstitution.
AIDS
When the immune system is damaged enough that significant opportunistic infections begin to develop, the person is considered to have AIDS. For surveillance purposes in the United States, a CD4 T-cell count less than 200/µL is also used as a measure to diagnose AIDS, although some opportunistic infections develop when CD4 T-cell counts are higher than 200/µL, and some people with CD4 counts under 200/µL may remain relatively healthy.
Many opportunistic infections and conditions are used to mark when HIV infection has progressed to AIDS. The general frequency of these infections and conditions varies from rare to common, but all are uncommon or mild in immunocompetent persons. When one of these is unusually severe or frequent in a person infected with HIV and no other causes for immune suppression can be found, AIDS can be diagnosed. [12]
Immunologic control of HIV
The primary mechanism for immunologic control of HIV appears to be CD8+ cytotoxic T-cells. T-cell responses are correlated with the steady-state viral load and hence, the rate of progression. [69] Cellular immunity is apparently responsible for some multiply-exposed, but uninfected individuals. [70, 71]
Although antibodies against HIV can be detected, it is clear that they are not sufficiently neutralizing to assist with immunologic control of the infection.
The role of NK (Natural Killer) cells may be important in the initial control of HIV. Escape mutations have been detected, implying that immunologic pressure on HIV exists from NK cells. [72]
Opportunistic infections and conditions
Even after starting therapy and with effective suppression of viral load, patients with persistently low CD4 counts remain at high risk for opportunistic infections. In general, all patients remain at a relatively high risk for opportunistic infections and other AIDS-related events for the first 6 months of antiretroviral therapy. [73] An observational study of 20,730 patients with HIV in Uganda found that, among patients with more than 6 months of follow-up after the initiation of antiretroviral therapy, the pre-therapy CD4 count was still predictive of mortality. [74]
Opportunistic infections and conditions include the following (*added in the 1993 AIDS surveillance case definition):
-
Candidiasis of bronchi, trachea, or lungs
-
Candidiasis, esophageal
-
Cervical cancer, invasive*
-
Coccidioidomycosis, disseminated or extrapulmonary
-
Cryptococcosis, extrapulmonary
-
Cryptosporidiosis, chronic intestinal (duration >1 mo)
-
Cytomegalovirus disease (other than liver, spleen, or nodes)
-
Cytomegalovirus retinitis (with vision loss)
-
Encephalopathy, HIV-related
-
Herpes simplex: chronic ulcer or ulcers (duration >1 mo) or bronchitis, pneumonitis, or esophagitis
-
Histoplasmosis, disseminated or extrapulmonary
-
Isosporiasis, chronic intestinal (duration >1 mo)
-
Kaposi sarcoma
-
Lymphoma, Burkitt (or equivalent term)
-
Lymphoma, immunoblastic (or equivalent term)
-
Lymphoma, primary, of the brain
-
Mycobacterium avium complex or Mycobacterium kansasii infection, disseminated or extrapulmonary
-
M tuberculosis infection, any site (pulmonary* or extrapulmonary)
-
Mycobacterium infection with other species or unidentified species, disseminated or extrapulmonary
-
Pneumocystis pneumonia
-
Pneumonia, recurrent*
-
Progressive multifocal leukoencephalopathy
-
Salmonella septicemia, recurrent
-
Toxoplasmosis of the brain
-
Wasting syndrome due to HIV infection
Although malaria is not typically considered an opportunistic infection, its incidence was found to be significantly higher among children in Tanzania that were perinatally infected with HIV than those without HIV infection. [75] This was true for physician-diagnosed clinical malaria, probable malaria involving laboratory testing for parasitemia, as well as malaria that was confirmed by blood smear.
There also appears to be an increased rate of anal cancer in high-risk groups (in particular, men who have sex with men). This is unsurprising considering the link between anal cancer and human papillomavirus (HPV), and the fact that cervical cancer, also caused by HPV, is considered an AIDS-defining condition. [76]
HIV encephalopathy is a severe condition usually seen in end-stage disease. Milder cognitive impairments may exist with less advanced disease. For example, one study found significant deficits in cognition, planning, coordination, and reaction times in HIV-infected compared to uninfected children, effects that were more pronounced in those with higher viral loads. [77]
Etiology
HIV disease is caused by infection with HIV-1 or HIV-2, both of which cause very similar conditions. They differ in transmission and progression risks.
Epidemiology
United States statistics
According to the Centers for Disease Control and Prevention (CDC), from 2013-2017, the estimated rate of HIV infection diagnosis in all 50 US states and the District of Columbia decreased. The annual number of diagnoses remained stable. In 2018, the rate was 11.4 per 100,000 population, [78] and 37,832 individuals were diagnosed with HIV infection that year. Numbers and rates of HIV infection increased in some subgroups and decreased in others. Variations in trends among groups are expected and may result from differences in testing behaviors, targeted HIV testing initiatives, and/or changes in the numbers of new HIV infections in some subgroups. [79]
From 2013-2017, the annual number and rate of HIV infections classified as stage 3 (AIDS) in the United States decreased. In 2018, the rate of infections classified as stage 3 (AIDS) was 5.2 per 100,000 population. The number and rate of deaths among persons with infection ever classified as stage 3 (AIDS) remained stable. In 2017, the rate of deaths in persons with stage 3 (AIDS) was 3.9 per 100,000 population. Deaths among persons with stage 3 (AIDS) may be due to any cause. [78]
US rates vary by state. See the latest CDC surveillance report for full details.
The overall figures may give a false impression that the HIV epidemic is relatively homogeneous. In fact, the HIV epidemic is best viewed as numerous separate epidemics among distinct risk groups, although the various epidemics clearly have some level of overlap. In any given area, the infection may be most prevalent among users of intravenous drugs who share needles. In another, the main risk group may be men who have sex with other men. And in yet another, the main risk group may be female sex workers.
These sub-epidemics each follow their own pattern, although there is some degree of interdependence. Early on, nearly all cases of HIV infection detected in the Western Hemisphere were in homosexual men, but the spread of the disease to female partners of bisexual men with HIV infection gave rise to an increased rate among heterosexual persons.
Contributing to the increased cross-prevalence were persons with hemophilia who had been infected with HIV from contaminated factor VIII concentrate and persons who used intravenous drugs, an activity that transcends all sexual preferences. In 2014, 70% of new HIV infections were reported in homosexual men, and infected heterosexual women outnumber infected heterosexual men nearly two to one. [79]
One community-based study targeting areas where men who have sex with men (MSM) meet demonstrated that an average of 44% of study participants appeared unaware of their HIV-positive status. High rates of positivity and unawareness of positive status were associated with younger participants, men of Black non-Hispanic race, and lower education levels.
Healthcare visits in the preceding year were associated with a lower rate of unawareness (37% vs 81%) but a higher rate of HIV-positivity (21% vs 12%). Because this study targeted a high-risk group and may involve participation bias, the overall rate of HIV infection (19%) cannot be easily extrapolated to the overall population. [80]
Mortality from HIV disease has not been among the 15 leading causes of death in the United States since 1997. The age-adjusted death rate for HIV disease peaked in 1995 at 16.3 per 100,000 population, decreased 69.9% through 1998, then further decreased 30.2% from 1999 through 2007, to 3.7 per 100,000 population. In 2007, a total of 11,295 persons died from HIV disease. However, HIV disease has remained among the five leading causes of death for specific age groups for females, and in the Black population. [81]
Adolescents and young adults
From 2013-2017, CDC HIV surveillance statistics show that rates among children (< 13 years) and persons aged 13 years to 24 years decreased. In 2018, the highest rate (32.4 per 100,000 population) of new HIV infections in the United States was in adults aged 25 years to 29 years, followed by adults aged 20 years to 24 years (27.6 per 100,000 population). Among all adults and adolescents, males accounted for 81% of new HIV infections. [78] The highest rates per 100,000 population were 39.3 in Blacks, followed by 16.2 in Hispanics/Latinos, 12.4 in persons of multiple races, 11.8 in native Hawaiians/other Pacific Islanders, 7.8 in American Indians/Alaskan natives, 4.9 in Whites, and 4.7 in Asians. Male-to-male sexual contact accounted for 72.1% (8,800 individuals) and 3% for male-to-male sexual contact and injection drug use. [78] The percentage of youths tested for HIV infection was 12.9% in high-school students and 34.5% in individuals aged 18 years to 24 years. Testing was lower in males than females. More than half (59.5%) of youths with HIV infection are unaware of their infection. [82]
International statistics
According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), [83] worldwide in 2018, approximately 37.9 million people were infected with HIV. UNAIDS estimates that approximately 1.7 million people were newly infected with HIV and that 770,000 people died of AIDS in 2018, both statistics showing a decline over time.
The vast majority of infections remain in sub-Saharan Africa, where 5.2% of the population is believed to be infected. Between 2004 and 2006, the prevalence of HIV infection in central and eastern Asia and Eastern Europe increased by 21%. During this period, the number of new HIV infections in persons aged 15-64 years rose by 70% in Eastern Europe and central Asia.
The infection rates in many developed countries remain stable, and some developing countries have achieved significant gains in controlling and even reversing the effects of the HIV epidemic. However, this is partially due to deaths in HIV-infected people, together with simultaneous prevention of new infections. India, for example, has used a national prevention campaign focusing on high-risk populations that may have prevented 100,000 new HIV infections over the 5 years it has been implemented, with increasing results seen in areas with higher levels of investment. [84] These figures together show that global HIV infection is in a state of flux.
Men who have sex with men (MSM) are still 28 times more likely than heterosexuals to contract HIV infection despite sharp declines of infection among such populations in Western countries. The institution of pre-exposure prophylaxis (PrEP) in Western Europe, North America, and Australia has dramatically decreased transmission rates among gay men in those areas. [85]
The mortality rate in some countries has greatly increased. In South Africa (a country that, despite having a relatively late-onset HIV epidemic, has developed one of the highest prevalence rates), the all-cause HIV-associated mortality rate increased by 79% between 1997 and 2004. In women aged 25-34 years, mortality rates increased by 500% during this period.
Swaziland has the highest overall prevalence of HIV infection (>26% of all adults based on 2007 figures).
The Ministry of Health in Zambia predicts that, without therapy and assuming current levels of prevalence, young adults have a 50% lifetime risk of dying from AIDS.
In developing nations, co-infection with HIV and tuberculosis is very common. The immunosuppressed state induced by HIV infection contributes not only to a higher rate of tuberculosis reactivation but also to an increased disease severity, as with many other opportunistic infections.
Further details of the global epidemic can be found in the UNAIDS Global HIV & AIDS statistics — 2019 fact sheet.
Racial, sexual, and age-related differences in incidence
In the United States, the rate of HIV infection is highest in Blacks (44.3 cases per 100,000 population). The prevalence is also high among Hispanic persons (16.4 per 100,000 population). [79] These increased rates result from socioeconomic factors rather than genetic predisposition.
In the developed world, HIV infection is much more common in males. In 2015, males accounted for 81% of all diagnoses of HIV infection among adults and adolescents in the United States. [79] Among heterosexuals, females are more likely to acquire HIV infection from an infected male than a male is from an infected female, but a large proportion of infections in males are due to homosexual contact, with or without injection drug use. Males are also more likely to acquire HIV infection from injection drug use alone.
Males were also more likely to acquire HIV infection through contaminated blood products for treatment of hemophilia before universal testing of the blood supply was instituted. The risk for HIV exposure from factor VIII concentrates has been virtually eliminated by viricidal treatment of plasma-derived factor VIII concentrates, as well as the introduction of recombinant factor VIII concentrates and the gradual elimination of albumin from the production process used for these products.
In the developing world, HIV infection is equally common in males and females. The primary route of HIV transmission in the developing world is heterosexual contact.
Young adults tend to be at higher risk of acquiring HIV, typically through high-risk activities such as unprotected sexual intercourse or intravenous drug use. In 2009 in the United States, the largest percentage (15% of all diagnoses) and the highest rate (36.9 per 100,000 population) were in persons aged 20 years to 24 years. [79]
Children may become infected by transplacental transmission or by breastfeeding. Rare cases of children infected after sexual abuse by HIV-infected adults have been reported.
Prognosis
The prognosis in patients with untreated HIV infection is poor, with an overall mortality rate of more than 90%. The average time from infection to death is 8-10 years, although individual variability ranges from less than 1 year to long-term nonprogression. Many variables have been implicated in HIV's rate of progression, including CCR5-delta32 heterozygosity, mental health, [86] concomitant drug or alcohol abuse, superinfection with another HIV strain, nutrition, and age.
There is less evidence that treatment of HIV-2 infection slows progression, and certain antiretroviral medications (specifically the non-nucleoside–analogue reverse-transcriptase inhibitors) are not effective against HIV-2. The HIV-1 viral-load assays are much less reliable at quantifying HIV-2, if they work at all. HIV-2 viral load assays have been developed, but none has been approved by the US Food and Drug Administration except as blood donor–screening tools.
Once infection has progressed to AIDS, the survival period is usually less than 2 years in untreated patients. Persons in whom the infection does not progress long-term may not develop AIDS for 15 years or longer, although many still exhibit laboratory evidence of CD4 T-cell decline or dysfunction. [87, 88, 89, 90]
The appropriate use of combination antiretroviral therapies and prophylaxis for opportunistic infections dramatically improves survival and greatly decreases the risk for secondary opportunistic infections. [91, 92, 93] The risk for AIDS-associated lymphoma is not altered by antiviral therapy and, as such, has grown in prevalence among overall AIDS-defining conditions.
Sackoff et al found that between 1999 and 2004, the HIV-related mortality rate in New York City decreased each year by approximately 50 deaths per 10,000 people with AIDS. The rate of non–HIV-related deaths also showed a decline, more modest but consistent, with about 7.5 fewer deaths per 10,000 people with AIDS per year. [92]
Importantly, many researchers have consistently shown that the primary risk factor for infection affects mortality. For example, the mortality rate among intravenous drug users tends to be higher, whether related to HIV disease or non-HIV disease.
Overall, with the increasing use of antiretroviral therapy and the introduction of better antiviral regimens, survival with HIV infection has increased over time, although it is not yet equivalent to that in uninfected individuals.
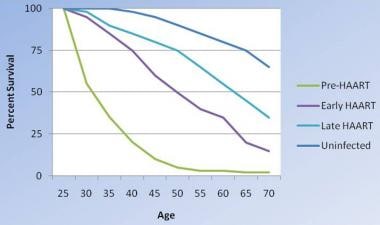 Changes in survival of people infected with HIV. As therapies have become more aggressive, they have been more effective, although survival with HIV infection is not yet equivalent to that in uninfected people. Modified from Lohse N et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87-95.
Changes in survival of people infected with HIV. As therapies have become more aggressive, they have been more effective, although survival with HIV infection is not yet equivalent to that in uninfected people. Modified from Lohse N et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87-95.
[94]
In addition to the concern for new opportunistic infections, pre-existing infections can reactivate and cause significant disease in people with AIDS. The most important example on a global scale is that of tuberculosis, as reactivated tuberculosis can cause symptomatic disease with lower levels of reactivation.
Other important pathogens include cytomegalovirus, (which causes retinitis, pneumonitis, and colitis) and Pneumocystis jiroveci (formerly known as Pneumocystis carinii; the causative organism in Pneumocystis pneumonia). In immunocompetent hosts, these organisms are generally nonpathogenic, and asymptomatic infection is common (and in the case of cytomegalovirus infection, life-long).
Antiviral medications are associated with adverse effects and thus contribute to patient morbidity and mortality rates, especially because of the growing population of long-term survivors who are receiving combination antiviral therapy. In particular, protease inhibitors may cause lipid-profile abnormalities.
In a study of 6,036 HIV-infected patients who had achieved suppression of HIV with antiretroviral therapy, researchers found that the incidence of non-Hodgkin lymphoma (NHL) remained high (171 per 100,000 person-years [PY]), far exceeding the rate of approximately 10 to 20 per 100,000 person-years reported in HIV-uninfected populations. The high incidence of NHL was observed even in patients with nadir CD4 cell count > 200 cells/μl (140 per 100,000 PY). After adjustment for older age, white race, male sex, HCV coinfection, and time-varying CD4 cell count, the risk for NHL was higher when HIV viremia was above the limit of detection (50 copies/mL) in a dose-dependent manner. [94, 95]
Patient Education
Patients with HIV infection should be counseled about the risks of infecting their sexual partners with HIV. Safer sex practices and treatment of concurrent sexually transmitted diseases, both in the patient and in sexual partners, considerably reduce the risk for transmission. Patients with HIV infection should be encouraged to inform their sexual partners of their status; failure to do so has resulted in successful prosecutions in several countries. Sexual contacts should be tested.
Some HIV-infected people actively seek out other persons with HIV infection for sex under the assumption that they are not putting themselves or anyone else at an increased risk. However, it is clear that co-infections with multiple HIV strains (whether the same or different clades) can and do occur, and that such events may result in a rapid deterioration of a previously stable infection. A growing number of new infections are drug resistant upon first presentation, suggesting that these infections were transmitted from individuals receiving therapy.
Higher viral loads in the source partner are associated with higher transmission rates; thus, because barrier contraception is imperfect (although by far the best method to prevent sexual transmission), good control of viral load is important.
Intravenous drug users should be counseled on the risks of sharing intravenous drug paraphernalia.
For patient education information, see the Infections Center and Sexual Health Center, as well as HIV/AIDS and Rapid Oral HIV Test.
-
Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC (Dr Edwin P Ewing, Jr).
-
Genome layout of human immunodeficiency virus (HIV)–1 and HIV-2.
-
Timeline of CD4 T-cell and viral-load changes over time in untreated human immunodeficiency virus (HIV) infection. Courtesy of Wikipedia (based on an original from Pantaleo et al (1993)).
-
Incidence of HIV infection by risk group. Courtesy of CDC (derived from the revised 2006 estimated figures).
-
Changes in survival of people infected with HIV. As therapies have become more aggressive, they have been more effective, although survival with HIV infection is not yet equivalent to that in uninfected people. Modified from Lohse N et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87-95.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- HAART Studies and DHHS Guidelines
- Prophylaxis for Opportunistic Infections
- Treatment of Opportunistic Infections
- Treatment of HIV-Associated Lipodystrophy
- Suppressive Therapy for Herpes Simplex Virus 2 Infection
- Treatment of HIV-Associated Diarrhea
- Deterrence and Prevention of HIV Infection
- Consultations
- Long-Term Monitoring
- Show All
- Guidelines
- Medication
- Medication Summary
- Antiretroviral agent, nucleoside reverse-transcriptase inhibitor
- Antiretroviral Agent, Protease Inhibitor
- Antiretroviral agent, non-nucleoside reverse-transcriptase inhibitor
- Antiretroviral Agent, Integrase Inhibitor
- Antiretroviral Agent, Fusion Inhibitor
- Antiretroviral agent, CCR5 antagonist
- HIV, Entry Inhibitors
- HIV, Attachment Inhibitors
- Capsid Inhibitors
- Complete Regimen Combinations
- Antiretroviral Combinations
- CYP3A4 Inhibitors
- Antibiotic, Sulfonamide Derivative
- Growth hormone releasing factor
- Show All
- Questions & Answers
- Media Gallery
- Tables
- References