RenovaCare Stockholder Update and 2020 Outlook from the CEO
/EIN News/ -- SCOTTSDALE, Ariz., Dec. 05, 2019 (GLOBE NEWSWIRE) -- RenovaCare, Inc. (Symbol: RCAR), developer of patented technologies for spraying self-donated stem cells for the regeneration of skin and other organs and tissues, is pleased to provide this update from its new CEO, Mr. Alan L. Rubino, as well as his outlook for 2020.
Letter from the CEO
Dear Valued Stockholders and Supporters:
During my 35 years of industry experience, I have led 8 large business units (over $1 billion in annual revenues) and presided over 20 new product launches.
Yet, the technology platform and patent portfolio for organ and tissue regeneration at RenovaCare (Symbol: RCAR) is one of the most compelling opportunities I have seen in the emerging cell therapeutics category.
Before sharing my outlook for 2020, I want to thank all our stakeholders for the confidence entrusted in me and my management team to push forward on our goal of potentially replacing painful skin grafting surgeries with a gentle healing mist of one’s own skin stem cells using our SkinGun™.
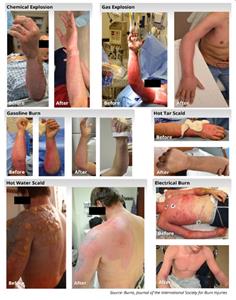
Our commitment to this mission is supported by peer-reviewed publications and the compelling treatment outcomes of more than 70 second-degree burn patients (see pictures below), who were experimentally treated with an early version of the technology underlying the SkinGun™.
Our Treatment Approach
The RenovaCare treatment approach is simple: the patient provides a skin sample, which can be as small as a postage stamp. This yields a large number of healthy skin stem cells, which are then sprayed back onto a patient’s burn or wound, in as little as 90 minutes.
Many of the SkinGun™ patients were discharged by physicians and able to leave the hospital in a matter of days. These patients had healed, or in medical terms, re-epithelialized.
In contrast, skin grafting patients require the removal of a large piece of healthy skin for surgical attachment onto their burned areas. They can remain hospitalized for weeks or months, may undergo multiple surgeries, and often endure ongoing physical therapy.
For second-degree burn patients, we envision our cell spray as a gentle, fast-healing, and scar-free therapeutic option.
Our approach is driven by less time in the operating room, shorter hospital stays, and less complex patient recovery. For hospitals and insurance providers, already burdened by rising healthcare costs, this may be a welcomed alternative to current treatment methodologies.
2017 – 2019 Highlights
The last few years have been exciting indeed, with the highlight of 2017 being the almost year-long display of the RenovaCare SkinGun™ at the Science Museum in London (UK), which holds a world-class collection of scientific, technological and medical achievements.
Along with the $15.5 million capital investment by a single investor, the Chairman of the Board of RenovaCare, much of the focus in 2018 and 2019 has continued to be on our regulatory program and planning for the clinical trials of the SkinGun™.
For me, 2019 was clearly a pivotal year for RenovaCare.
We were awarded new patent protections, allowing our novel SkinGun™ to now spray all varieties of tissues and cells, thus opening the door for its potential application in the regeneration of tissues and organs, beyond skin.
“Just as we’ve used needles to inject drugs and vaccines in the 20th century, the syringe of tomorrow could be our patented SkinGun™, spraying a gentle healing mist of self-donated stem cells to regenerate human organs and tissue,” stated Dr. Ben Walthall, former Johnson & Johnson R&D Worldwide Director of Surgical Wound Care and Tissue Regeneration, and currently RenovaCare VP, Regenerative Technologies.
New Additions to Our Team
During 2019, we also bolstered our organization with the addition of leading medical, regulatory, manufacturing, clinical, and business professionals with deep expertise. This reflects not only growing clinical and medical confidence in our regulatory (FDA) pathway, but also in our technology platform.
Here are just a few recent appointments to our Management Team and Advisory Board:
Dr. Robin A. Robinson – VP Scientific Affairs – a respected authority on the development of breakthrough biomedical technologies, for which he was cited in 2018 as one of the top 100 innovators in medicine by The Medicine Maker.
Dr. Robinson was appointed as Director of the Biomedical Advanced Research and Development Authority (BARDA), with an annual budget of $1.35 billion and a staff of 250 scientists and medical experts.
He has successfully established over 80 commercial partnerships, including with the Gates Foundation, Wellcome-Trust, Sanofi, GlaxoSmithKline plc, Novartis, Merck, Roche/Genentech, Amgen, Johnson & Johnson (Crucell), SIGA, Regeneron and Emergent.
Dr. Robinson has also developed collaborations with universities and foreign governments, including the Johns Hopkins University, North Carolina State University, and the United Kingdom, Canada, Australia, France, Germany, and others.
Dr. Benjamin Walthall – VP Regenerative Technologies – over a career spanning almost four decades, Dr. Walthall served as R&D Worldwide Director for Johnson & Johnson, Surgical Wound Care, where he was responsible for technology strategy, portfolio management and product development programs.
While at Johnson and Johnson, he also served as Worldwide Director R&D of Tissue Regeneration and previously as Worldwide Director R&D Advanced Wound Care.
In these roles, Dr. Walthall created new tissue regeneration business and developed technologies to clinically utilize novel tissue engineering scaffold for skin repair and advanced wound care.
Dr. Emanual Maverakis – Advisor – a professor at the University of California Davis School of Medicine, holding appointments in the Department of Dermatology and the Department of Medical Microbiology and Immunology. He is the recipient of numerous honors, including a Presidential Early Career Award for Scientists and Engineers, which is the highest honor bestowed by the United States government for outstanding scientists and engineers.
Dr. Maverakis has also received the National Institutes of Health Director’s New Innovator Award, a Career Award for Medical Scientists from the Burroughs Welcome Fund, and a Physician-Scientist Career Award from the Howard Hughes Medical Institute. In 2019, he was elected as a fellow of the California Academy of Sciences.
For the backgrounds of our entire team, please visit our website at www.renovacareinc.com, where you can also sign up to late breaking news via email or through social media.
Looking Forward to 2020
We enter a brand-new year excited by the many opportunities that lay before us with ample cash (approximately $13 million) and the support and encouragement of our many stakeholders.
Our highest priority in 2020 will be to advance the SkinGun™ through our regulatory program so we can obtain approval for conducting clinical trials, initially for establishing the safety of our device.
While there are no guarantees of obtaining regulatory consent for our clinical trials, our confidence is strengthened by peer-reviewed publications and by the compelling visual outcomes of the more than 70 burn patients (see pictures below) experimentally treated with an early version of the technology underlying the SkinGun™.
Our goal is to bring our therapy to the tens of millions worldwide who suffer from burns, chronic and acute wounds, and scars, an estimated $45 billion market in the U.S. alone.
We Are Grateful for Your Support
With your continued confidence and trust, we remain steadfast in our mission to potentially replace painful skin grafts with, we believe, the greatest breakthrough in wound healing in the past half-century – a gentle mist of skin stem cells from our SkinGun™.
Of course, we also look forward to investigating the SkinGun™ for spraying a variety of tissues and cells, thus opening the door for its potential application in the regeneration of tissues and organs, beyond burns.
Finally, if you haven’t signed up to receive emails on our latest news and ongoing advances, I urge you to go to www.renovacareinc.com and sign-up now.
On behalf of our entire team, thank you again for your support and belief in our goals.
From all of us here at RenovaCare, we wish you a healthy and prosperous 2020.
Sincerely,
Alan L. Rubino
Chief Executive Officer & President
RenovaCare, Inc.
IMPORTANT NOTICES AND DISCLAIMERS
RenovaCare products are currently in development. They are not available for sale in the United States. There is no assurance that the Company’s planned or filed submissions to the U.S. Food and Drug Administration will be accepted or cleared by the FDA.
Legal Notice Regarding Forward-Looking Statements
No statement herein should be considered an offer or a solicitation of an offer for the purchase or sale of any securities. This release contains forward-looking statements that are based upon current expectations or beliefs, as well as a number of assumptions about future events. Although RenovaCare, Inc. (the “Company”) believes that the expectations reflected in the forward-looking statements and the assumptions upon which they are based are reasonable, it can give no assurance that such expectations and assumptions will prove to have been correct. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “could,” “expect,” “anticipate,” “estimate,” “believe,” “want,” “intend,” “our goal,” “our mission,” or “project” or the negative of these words or other variations on these words or comparable terminology. The reader is cautioned not to put undue reliance on these forward-looking statements, as these statements are subject to numerous factors and uncertainties, including but not limited to: the timing and success of clinical and preclinical studies of product candidates, the potential timing and success of the Company’s product programs through their individual product development and regulatory approval processes, adverse economic conditions, intense competition, lack of meaningful research results, entry of new competitors and products, inadequate capital, unexpected costs and operating deficits, increases in general and administrative costs, termination of contracts or agreements, obsolescence of the Company’s technologies, technical problems with the Company’s research, price increases for supplies and components, litigation and administrative proceedings involving the Company, the possible acquisition of new businesses or technologies that result in operating losses or that do not perform as anticipated, unanticipated losses, the possible fluctuation and volatility of the Company’s operating results, financial condition and stock price, losses incurred in litigating and settling cases, dilution in the Company’s ownership of its business, adverse publicity and news coverage, inability to carry out research, development and commercialization plans, loss or retirement of key executives and research scientists, and other risks. There can be no assurance that further research and development will validate and support the results of our preliminary research and studies. Further, there can be no assurance that the necessary regulatory approvals will be obtained or that the Company will be able to develop commercially viable products on the basis of its technologies. In addition, other factors that could cause actual results to differ materially are discussed in the Company’s most recent Form 10-Q and Form 10-K filings with the Securities and Exchange Commission. These reports and filings may be inspected and copied at the Public Reference Room maintained by the U.S. Securities & Exchange Commission at 100 F Street, N.E., Washington, D.C. 20549. You can obtain information about operation of the Public Reference Room by calling the U.S. Securities & Exchange Commission at 1-800-SEC-0330. The U.S. Securities & Exchange Commission also maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the U.S. Securities & Exchange Commission at http://www.sec.gov. The Company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Social Media Disclaimer:
Investors and others should note that we announce material financial information to our investors using SEC filings and press releases. We use our website and social media to communicate with our subscribers, shareholders and the public about the company, RenovaCare, Inc. development, and other corporate matters that are in the public domain. At this time, the company will not post information on social media that could be deemed to be material information unless that information was distributed to public distribution channels first. We encourage investors, the media, and others interested in the company to review the information we post on the company’s website and the social media channels, including LinkedIn, Facebook and Twitter.
Contacts: RenovaCare, Inc.
Amit Singh, 888-398-0202
https://www.renovacareinc.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/23fa8e7c-92e5-4bc8-ba7f-dc47ced41b06
https://www.globenewswire.com/NewsRoom/AttachmentNg/4b95e49c-48fb-428d-a802-25f4f5fedd95
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.