3D Systems Receives FDA Clearance for Vantage® Ankle PSI – Expands VSP® Surgical Planning Applications in Partnership with Exactech
- Patient-specific guides require less procedural steps, reduce OR time for total ankle replacement surgeries
- Jointly developed end-to-end solution through collaboration with Exactech, which makes joint replacement implants, instruments, and technologies
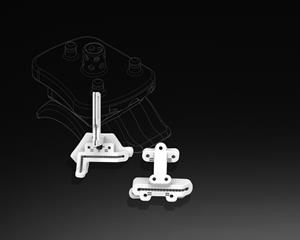
/EIN News/ -- ROCK HILL, S.C., Nov. 17, 2020 (GLOBE NEWSWIRE) -- 3D Systems (NYSE:DDD) today announced the Food and Drug Administration (FDA) has provided 510(k) clearance for the Vantage® Ankle PSI - its patient-specific total ankle surgical planning and 3D printed instruments. The product includes pre-surgical planning and a patient-specific 3D-printed instrument set that guides resections in the tibia and talus for total ankle replacement surgery using Exactech’s Vantage Total Ankle System. Vantage Ankle PSI increases operating room efficiency, reliability, and improves soft-tissue preservation around the joint. This innovation is a result of the collaboration between 3D Systems and Exactech (Gainesville, Florida), a developer and producer of innovative implants, instrumentation, and computer-assisted technologies for joint replacement surgery.
Patient-specific orthopaedic instruments are an enabling technology that help surgeons prepare the skeletal anatomy to receive an implant. The Vantage Ankle PSI product, the only solution to facilitate direct patient-specific osteotomies in the ankle, is designed to increase surgical efficiency by allowing the surgeon to reduce the number of steps required to prepare the anatomy with a patient-matched 3D-printed instrument set. Features unique to the product include a large footprint that helps to reliably seat the guide on the bone anatomy, improved visibility to alignment, and a corrugated design on the cutting slots that aid surgical irrigation. This is complemented by soft tissue offsets which are designed to preserve the periosteum, the outer fibrous layer of the bone which aids in its healing and recovery.
“3D Systems was founded on a spirit of innovation, and we are constantly looking for opportunities to expand the applications that can be addressed with our VSP® surgical planning solutions to benefit the medical community,” said Menno Ellis, EVP, healthcare solutions, 3D Systems. “Through our collaboration with Exactech, we drew upon our collective expertise in orthopaedics to develop an end-to-end solution for total ankle replacements that is unique to the market. The combination of pre-surgical planning and 3D-printed, patient-specific instruments allows the surgeon to visualize the patient anatomy and surgical approach in three dimensions, and then perform the surgery more efficiently with improved surgical outcomes.”
3D Systems is recognized as a pioneer in the personalized medicine space. The Company has manufactured more than 1 million medical device implants and supports 85+ CE-marked and FDA-cleared products. 3D Systems’ VSP surgical planning solutions include a service-based approach to personalized surgery, combining expertise in medical image processing, surgical planning, and 3D printing. The company has a long history in surgical planning with more than 10 years of expertise in craniomaxillofacial applications. To date, 3D Systems has worked with surgeons to plan and guide more than 140,000 patient-specific procedures.
“Our collaboration with 3D Systems represents a meaningful advancement that will accelerate the success of our Vantage Ankle prosthesis,” said Exactech CEO Darin Johnson. “Their expertise in surgical planning, as well as medical device design, regulatory, and manufacturing was invaluable in developing this patient-specific solution. The Exactech team is eager to bring this solution to market to simplify ankle arthroplasty’s surgical technique and enhance the patient and surgeon experience.”
3D Systems and Exactech have entered into a distribution agreement for the Vantage Ankle PSI product offered exclusively with Exactech’s Vantage Total Ankle System. The Vantage Ankle PSI is currently in pilot launch with full market availability expected in late 2021. Interested surgeons can contact their Exactech sales representative directly, visit Exactech’s website or join a live webinar on December 1, 2020 to learn more about this product.
Forward-Looking Statements
Certain statements made in this release that are not statements of historical or current facts are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the company to be materially different from historical results or from any future results or projections expressed or implied by such forward-looking statements. In many cases, forward-looking statements can be identified by terms such as "believes," "belief," "expects," "may," "will," "estimates," "intends," "anticipates" or "plans" or the negative of these terms or other comparable terminology. Forward-looking statements are based upon management’s beliefs, assumptions, and current expectations and may include comments as to the company’s beliefs and expectations as to future events and trends affecting its business and are necessarily subject to uncertainties, many of which are outside the control of the company. The factors described under the headings "Forward-Looking Statements" and "Risk Factors" in the company’s periodic filings with the Securities and Exchange Commission, as well as other factors, could cause actual results to differ materially from those reflected or predicted in forward-looking statements. Although management believes that the expectations reflected in the forward-looking statements are reasonable, forward-looking statements are not, and should not be relied upon as a guarantee of future performance or results, nor will they necessarily prove to be accurate indications of the times at which such performance or results will be achieved. The forward-looking statements included are made only as of the date of the statement. 3D Systems undertakes no obligation to update or review any forward-looking statements made by management or on its behalf, whether as a result of future developments, subsequent events or circumstances or otherwise.
About 3D Systems
More than 30 years ago, 3D Systems brought the innovation of 3D printing to the manufacturing industry. Today, as the leading Additive Manufacturing solutions partner, we bring innovation, performance, and reliability to every interaction - empowering our customers to create products and business models never before possible. Thanks to our unique offering of hardware, software, materials, and services, each application-specific solution is powered by the expertise of our application engineers who collaborate with customers to transform how they deliver their products and services. 3D Systems’ solutions address a variety of advanced applications in Healthcare and Industrial markets such as Medical and Dental, Aerospace & Defense, Automotive, and Durable Goods. More information on the company is available at www.3dsystems.com.
Investor Contact: investor.relations@3dsystems.com
Media Contact: press@3dsystems.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/05348b3c-2bcd-4cc0-b47b-b391ddaba614
Distribution channels: IT Industry, Media, Advertising & PR
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release