Patient Monitoring Devices Market to Hit USD 93.53 Bn by 2032, Fueled by Tech Advancements & Rising Healthcare Demand
"Expanding Innovations and Rising Demand Drive Growth in the Global Patient Monitoring Devices Market"
AUSTIN, TX, UNITED STATES, December 19, 2024 /EINPresswire.com/ --
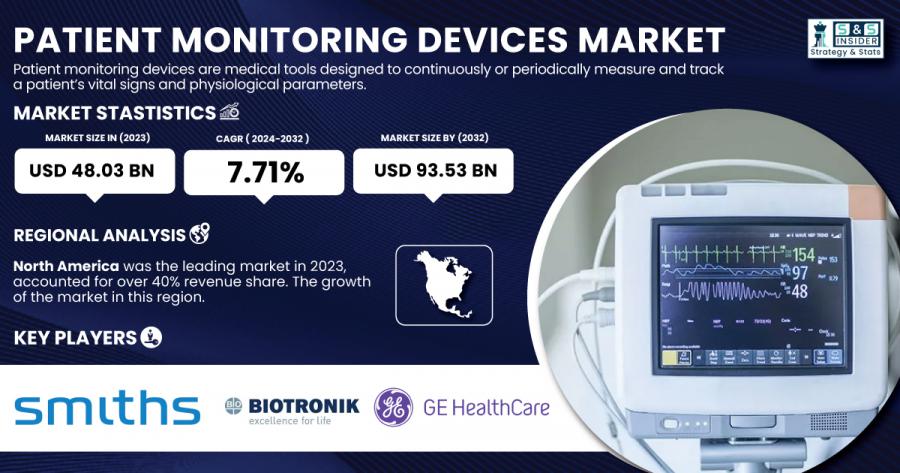
The Patient Monitoring Devices Market was valued at USD 48.03 billion in 2023 and is expected to reach USD 93.53 billion by 2032, growing at a CAGR of 7.71% over the forecast period 2024-2032. The rise in chronic diseases, an aging global population, and advances in remote monitoring technology are the primary drivers of this market’s growth. Additionally, the increasing demand for healthcare services, particularly in emergency care and home healthcare, is propelling the development of advanced patient monitoring solutions.
The market for patient monitoring devices is expanding due to the increasing demand for real-time healthcare management, the prevalence of chronic diseases, and the growth of home healthcare solutions. Technological advancements in wearable devices and remote monitoring tools further support this trend.
Market Overview
The Patient Monitoring Devices Market plays a crucial role in modern healthcare by enabling continuous tracking of vital signs, ensuring early detection of health issues, and improving patient outcomes. With healthcare providers increasingly focusing on personalized and timely care, the demand for these devices has surged. Monitoring devices are integral in various healthcare settings, including hospitals, ambulatory surgical centers, and home care environments.
The market is driven by advancements in technology that have made these devices more affordable, portable, and user-friendly. This is particularly important as healthcare providers transition toward value-based care, which emphasizes continuous patient monitoring, especially for individuals with chronic conditions like diabetes, cardiovascular diseases, and respiratory disorders. In 2023, the segment of multi-parameter monitoring devices dominated the market with a significant revenue share of 21%. These devices offer integrated solutions that monitor multiple vital signs like heart rate, oxygen saturation, and blood pressure.
Additionally, there is a growing focus on wearable monitoring devices that allow patients to be continuously monitored outside of healthcare facilities, helping reduce hospital readmissions and enabling more proactive care management.
Book Your Sample Report @ https://www.snsinsider.com/sample-request/4455
Segment Analysis
By Product:
The multi-parameter patient monitoring devices segment was the dominated segment in 2023, capturing a substantial market share of 29.5%. This segment is expected to continue its dominance over the forecast period due to the increasing adoption of devices that provide real-time monitoring of multiple vital signs such as ECG, blood pressure, and respiratory rate. These devices are compact, battery-operated, and low-cost, making them suitable for both hospital and home care environments. Their integration with mobile health apps and wireless connectivity options further enhances their appeal, especially in home healthcare settings where monitoring patients remotely is becoming more common.
By End-Use:
Hospitals
Hospitals remained the largest end-user of patient monitoring devices, accounting for over 49.6% of the market share in 2023. The need for precise and fast disease detection is critical in hospital environments, particularly in intensive care units (ICUs) and general wards, where patients require continuous monitoring. Hospitals use multi-parameter monitors to track vital signs such as heart rate, blood pressure, respiratory rate, and oxygen saturation, especially for patients with chronic illnesses or those undergoing surgery. The increasing volume of medical procedures and patient admissions related to chronic diseases and injuries further drives the demand for these devices in hospital settings.
Key Players:
• Smiths Medical
• Biotronik
• Mindray Medical International Ltd.
• Koninklijke Philips N.V.
• Nihon Kohden
• Welch Allyn
• Health anywhere Inc.
• Intel
• GE Healthcare
• Medtronic
• Bosch
• MASIMO CORPORATION
Ask For Enquiry @ https://www.snsinsider.com/enquiry/4455
Regional Analysis
In 2023, North America held the largest market share in the Patient Monitoring Devices Market, accounting for over 40.0% of the total revenue. This can be attributed to the high adoption rates of advanced healthcare technologies, strong healthcare infrastructure, and the presence of major healthcare device manufacturers such as GE HealthCare and Abbott Laboratories. The U.S. healthcare system is highly digitized, which supports the growth of patient monitoring technologies, especially in hospitals and home care settings.
Europe is expected to grow at a steady pace, supported by high healthcare expenditures and the increasing use of telemedicine and remote monitoring technologies. The adoption of these devices in countries like Germany, France, and the UK is rising due to government initiatives aimed at improving healthcare accessibility and efficiency.
The Asia Pacific region is anticipated to experience the fastest growth over the forecast period, driven by rapid healthcare infrastructure development, rising chronic disease prevalence, and the increasing demand for cost-effective monitoring solutions. Countries like China and India are seeing a surge in demand for affordable and portable patient monitoring devices as the healthcare system evolves to meet the needs of a large and aging population.
Ask For Buy @ https://www.snsinsider.com/checkout/4455
Recent Developments in Patient Monitoring Devices
• In April 2023, GE HealthCare received FDA clearance for its CARESCAPE Canvas Patient Monitoring Platform. This platform, along with CARESCAPE ONE, offers a modular system that can scale up its monitoring features to match the severity of a patient’s condition, improving both patient outcomes and healthcare efficiency.
• In January 2023, Senet and Telli Health introduced a new LoRaWAN-based remote patient monitoring hardware. This device allows healthcare providers to monitor patients in remote locations, particularly in underserved regions and indigenous communities. By leveraging low-power wide-area network (LPWAN) technology, the device ensures reliable communication in areas with limited infrastructure.
• In June 2022, Abbott received FDA approval for its FreeStyle Libre 2 device, an integrated continuous glucose monitor (iCGM) for diabetes management. The device provides real-time glucose monitoring, alerting patients to changes in their glucose levels and improving diabetes management for both adults and children.
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Our staff is dedicated to giving our clients reliable information, and with expertise working in the majority of industrial sectors, we're proud to be recognized as one of the world's top market research firms. We can quickly design and implement pertinent research programs, including surveys and focus groups, and we have the resources and competence to deal with clients in practically any company sector.
Office No.305-B, Arissa Avenue, Fountain Road, Kharadi, Pune, Maharashtra 411014
Akash Anand
SNS Insider | Strategy and Stats
+1 415-230-0044
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release