Vidac Pharma receives reinvestment from shareholders to drive clinical trials of its cancer drug candidates
More than 90% of owners participated in the AGM, showing solid commitment to Vidac. Vidac initiates preparation for Phase 2b study in skin cancer.
Vidac pharma Holding Plc (XSTU:SYM: T9G; ISIN: GB00BM9XQ619; WKN: A3DTUQ)
• Vidac initiates preparation for Phase second 2b study in skin cancer: will kick off regulatory preparations, contract with CRO signed.
Vidac Pharma Holdings Plc. (Hamburg and Stuttgart: T9G; ISIN:GB00BM9XQ619; WKN: A3DTUQ), a clinical-stage oncology biopharmaceutical company pioneering a novel class of cancer treatments, has raised extra capital of more than €600,000 from existing investors and management, ear-marked for the development of its two novel cancer drug candidates. Vidac will use the funds to drive a series of clinical trials, including a second Phase 2b clinical trial for VDA-1102 in advanced actinic keratosis (AK), an early form of skin cancer that can develop into cutaneous squamous cell carcinoma (CSCC). Vidac is initiating regulatory preparation for this trial, and has also signed a contract with Forschungsdock CRO, a German CRO that will help execute the trial. The principal investigator is Prof Dr Thomas Dirschka, one of the world’s pre-eminent experts in AK, and head of the world-leading dermatology research institute CentroDerm GmbH.
“I am thrilled by this show of confidence, which puts us in better shape to continue the development of our drugs in onco-dermatology and solid tumors,” Chief Executive Officer Max Herzberg said after the shareholder meeting. “I want to thank our Board, advisors, team and shareholders for their support for our entirely new approach in oncology, with drugs that reverse the supercharged metabolism of cancer cells. We will continue apace with testing of our two therapeutic candidates, including the extremely promising VDA-1275.”
The new Phase 2b trial with VDA-1102 will be with patients with advanced AK, as an earlier Phase 2b successful trial found higher sensitivity to the candidate drug in these patients at risk. The study will focus on highly proliferative lesions, which are the most likely to result in CSCC. Because of VDA-1102’s specific mode of action, it does not affect healthy cells, causing a minimum of side-effects.
Separately, VDA-1102 was shown to be both safe and efficacious in treatment of cutaneous T-cell lymphoma, for which Vidac has concluded a Phase 2a proof of concept trial, results of which it will report shortly. Vidac’s newest therapeutic candidate, VDA-1275, is now in advanced pre-clinical studies.
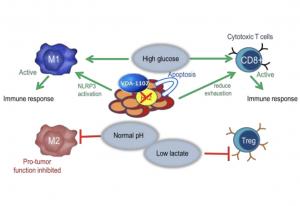
Both Vidac Pharma’s product candidates disrupt the interaction between the hexokinase 2 (HK2) isozyme and the voltage-dependent anion channels (VDACs) in mitochondria. Cancer cells overexpress HK2, which catalyzes the first step of the glucose metabolism, necessary to fuel tumor growth. HK2 blocks the channels, which prevents programmed cell death (apoptosis), supports cancer cell proliferation, and suppresses immune responses. The resulting high concentrations of lactate lead to an acidic and low-oxygen micro-environment in the cancer cells and the nearby tumor microenvironment, which fosters cancer growth. Clinical data for Vidac’s first-generation metabolic checkpoint modulator candidates have shown effects in halting cancer cell proliferation and restoring immune-sensitivity and apoptosis
Max Herzberg
Vidac pharma Holding Plc
72544257381
email us here
Visit us on social media:
LinkedIn
Distribution channels: Business & Economy, Chemical Industry, Companies, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release